Are you looking for an answer to the topic “How do you convert 20 grams of water to moles?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Therefore, 20g of water =20/18=1. 11 mole.Therefore, 20g of water =20/18=1. 11 mole.(b) 20 g of water. = 18 g.

Table of Contents
How do you convert 20g of water to moles?
Therefore, 20g of water =20/18=1. 11 mole.
What is the molar mass of 20 gram of water?
(b) 20 g of water. = 18 g.
How To Convert Grams To Moles – VERY EASY!
Images related to the topicHow To Convert Grams To Moles – VERY EASY!

How many moles is 25 grams of water?
As in the given question we have to calculate how many moles are present in the 25 grams of water and for this we have to divide the given weight of water by the molecular weight / mass of water. Hence, 1.38 moles (mol) are present in 25 grams of water.
How many moles are in 16g of water?
Answer. 16g of water is equal to 8/9 or 0.88.. moles.
How many moles are in 18 grams of water?
– We can say that 18 grams of water means same as 1 mole of water , and in this NA Avogadro number of water molecules are present , which means 6.02×1023 molecules.
How do you calculate moles of water?
Explanation: One mole of H2 O is made up of 2 moles of Hydrogen atoms and 1 mole of Oxygen atom. Mass of two moles of Hydrogen atoms = 2x 1 g/mol = 2 g/mol. Mass of one mole of water = 2 g/mol + 16 g/mol = 18 g/mol.
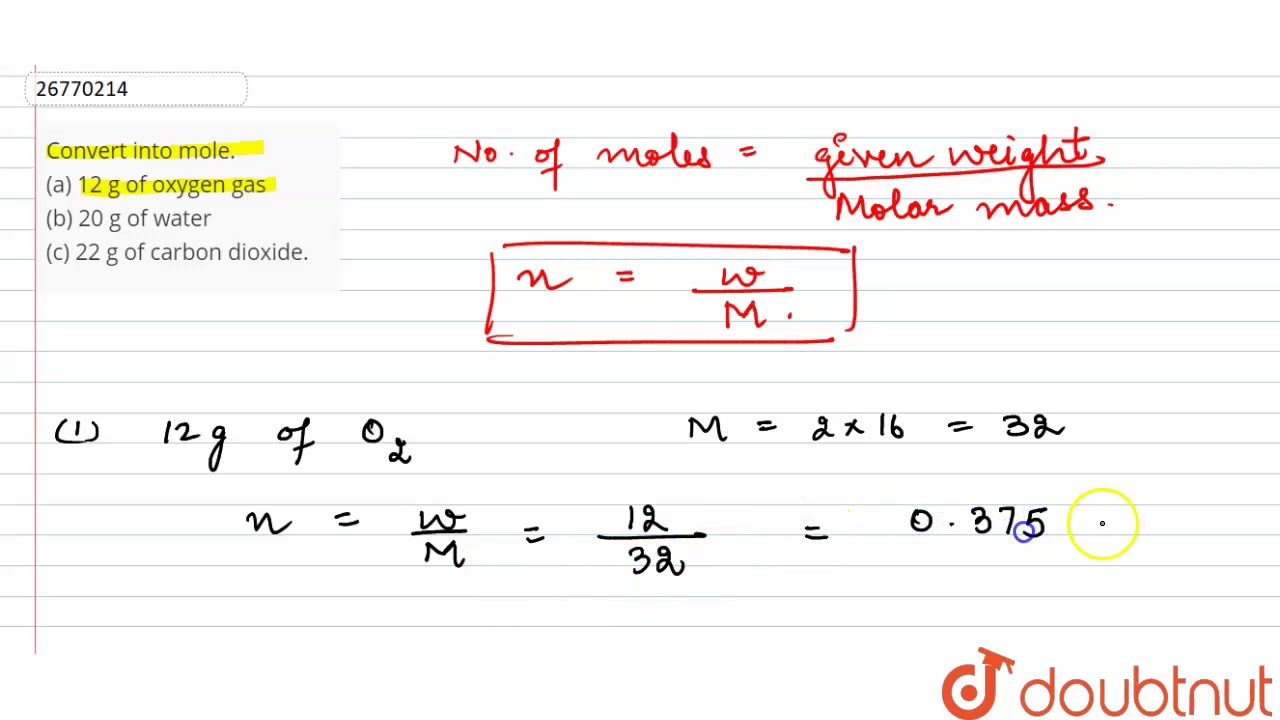
How do you convert 12g of oxygen to moles?
Therefore, 12 g of oxygen gas has 0.375 moles.
See some more details on the topic How do you convert 20 grams of water to moles? here:
Convert into mole20g of water – BYJU’S
20 g of water into mole molar mass of water(H2O) = 2 x H + O = 2 x 1g/mol + 16g/mol = 18 g/mol. Given mass = 20 g molar mass = 18g/mol
Convert grams Water to moles – Conversion of Measurement …
Do a quick conversion: 1 grams Water = 0.055508435061792 mole using the molecular weight calculator and the molar … 20 grams Water to mol = 1.11017 mol.
Convert 20g of water into moles class 11 chemistry CBSE
When $20{{g}}$ of water is converted, we get $1.11{{mol}}$. Additional information: Mole concepts enable us to solve stoichiometric problems involving mass …
Grams to Moles Calculator
Find the molar mass of a water molecule (H₂O). · Convert the volume of the water to its mass, assuming …
How do you convert 22 grams of CO2 to moles?
So, 22 gm of CO2 = 4422 moles of CO2 = 0.5 moles of CO2.
What is molar mass of water?
Convert into mole. (a) 12 g of oxygen gas (b) 20 g of water (c) 22 g of carbon dioxide….
Images related to the topicConvert into mole. (a) 12 g of oxygen gas (b) 20 g of water (c) 22 g of carbon dioxide….
How many moles are in 20 grams?
Therefore, 20g of water =20/18=1. 11 mole.
How many moles are there in 28g of water?
Answer. answer: 2 moles.
How many moles are in 27 grams of water?
Answer and Explanation: There are 1.5 moles of water in a 27 gram sample of water. The molar mass of water is 18.02 gmol g m o l .
How many grams are in 1 mole?
…
= 3 x 28.01 = 84.03 grams.
| FORMULAS Related Links | |
|---|---|
| Variance And Standard Deviation Formula | Equation Of A Tangent To A Circle |
How many moles are 3.6 gram of water?
Hence, the number of moles present in 3.6 gram of water are 0.2 moles.
How many moles are in 50 grams of water?
Expert-verified answer
The number of moles in 50gm of Water (H20) is 2.77 moles.
How many moles are there in 90g of water?
The correct answer is 5. The number of moles present in 90 g of water is 5.
How many grams of water are in a mole?
Find the molar mass of a water molecule (H₂O). It’s ca. 18.015 g/mol .
Convert into mole. (a) 12 g of oxygen gas (b) 20 g of water (c) 22 g of carbon dioxide
Images related to the topicConvert into mole. (a) 12 g of oxygen gas (b) 20 g of water (c) 22 g of carbon dioxide

How many moles are in 50 grams?
To calculate the weight of an element in grams to moles, we should know the molar mass of an element. For example, 50 grams of oxygen is equal to 3 moles.
How did you convert moles to grams?
The amount of grams in a mole depends on the substance you have. To work it out, find the atomic or molecular mass of your substance and multiply it by the number of moles you have. For one mole, the atomic or molecular mass will be the same as the weight.
Related searches to How do you convert 20 grams of water to moles?
- calculate the number of aluminium ions present in 0 051 gram of aluminium oxide
- 20 gram water
- how do you convert 20 grams of water to moles of nacl
- how do you convert 20 grams of water to moles of cuso4
- convert into mole 12 gram of oxygen gas
- moles to grams converter
- how many grams are equivalent to 20 moles of water
- how many moles in a gram
- how many grams are in 5 5 moles of li2o
- how do you convert 20 grams of water to moles of anhydrous kal(so4)2
- mol calculator
- how do you convert 20 grams of water to moles of water
- what is the mass of 0 5 mole of water molecules
- how many grams are in 5.5 moles of li2o?
- how do you convert 20 grams of water to moles calculator
- how do you convert 20 grams of water to moles of copper
- moles to moles calculator
Information related to the topic How do you convert 20 grams of water to moles?
Here are the search results of the thread How do you convert 20 grams of water to moles? from Bing. You can read more if you want.
You have just come across an article on the topic How do you convert 20 grams of water to moles?. If you found this article useful, please share it. Thank you very much.