Are you looking for an answer to the topic “How do you find the number of spectral lines in hydrogen spectrum?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
A hydrogen atom has 6 spectral lines. Spectral emissions occurs when an electron transitions jumps from a higher energy state to a lower energy state. The spectral lines are grouped into series according to the lower energy level.If an atom has N=number of levels then the number of transitions and therefore number of spectral lines is N−1. However, for energy levels in an atom it is common to use three numbers to label each energy levels.Hence there are 10 transitions and hence 10 spectral lines possible.

Table of Contents
How do you find the number of spectral lines in a hydrogen atom?
A hydrogen atom has 6 spectral lines. Spectral emissions occurs when an electron transitions jumps from a higher energy state to a lower energy state. The spectral lines are grouped into series according to the lower energy level.
How do you determine the number of spectral lines?
If an atom has N=number of levels then the number of transitions and therefore number of spectral lines is N−1. However, for energy levels in an atom it is common to use three numbers to label each energy levels.
Trick To Find Number Of Spectral Lines || Atomic Structure || Hydrogen Spectrum #neet
Images related to the topicTrick To Find Number Of Spectral Lines || Atomic Structure || Hydrogen Spectrum #neet

How many spectral lines are produced in the spectrum of hydrogen?
Hence there are 10 transitions and hence 10 spectral lines possible.
What is the maximum number of spectral lines emitted by a hydrogen?
6-number of lines are emitted.
How do you find maximum spectral lines?
The maximum number spectral lines possible is 4. 3 spectral lines are obtained for the transition 4→3→2→1 and one spectral line is obtained for any one of the following transition 4→1 or 4→3→1 or 4→2→1.
How many spectral lines are in the emission spectrum?
The electron energy level diagram for the hydrogen atom. He found that the four visible spectral lines corresponded to transitions from higher energy levels down to the second energy level (n = 2). This is called the Balmer series.
What is the number of possible spectral lines of electron?
There are 6 possibilities.
See some more details on the topic How do you find the number of spectral lines in hydrogen spectrum? here:
What is the number of spectral lines obtained on a … – Quora
To calculate number of spectral lines From nth energy level to ground state n(n-1)/2 And from n2 energy level to n1 energy level (n2-n1)x(n2-n1+1)/2 So if …
5.7: Spectral Lines of Atomic Hydrogen – Chemistry LibreTexts
Spectral Lines of Hydrogen Energy levels are designated with the variable n. The ground state is n=1, the first excited state is n=2, and so on. The energy …
Hydrogen spectral series – Wikipedia
The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula.
Number of spectral line in hydrogen atom is A 6 B 8 class 12 …
The number of spectral lines in the spectrum of the hydrogen atom depends on the number of the energy level from which the electron is jumping and the manner in …
2.19-Determine no.of spectral lines in hydrogen atom / atomic structure
Images related to the topic2.19-Determine no.of spectral lines in hydrogen atom / atomic structure

How many spectral lines are formed in hydrogen spectrum when excited electrons in a sample of hydrogen jump from 5th orbit to 1st in multiple steps?
So, the six spectral lines are, 5 → 4.
How many spectral lines will be visible in emission spectrum of H atom if electrons transition are from 5th excited state to ground state?
A certain transition in H spectrum from an excited state to ground state in one or more steps gives rise to a total of 10 lines.
How many number of spectral lines will appear if an electron jumps from 4th to 2nd energy level?
Thus, 5→2,4→2,3→2 it shows three lines lie in the visible region.
What is the maximum number of spectral lines emitted by a hydrogen atom when it is in the third excited state All India?
Solution : There will be 6 spectral lines corresponding to transitions. Third excited state means n = 4.
What is the maximum number of spectral lines that can be formed when an electron is transmitted from 6th orbit to 3rd?
Hence, the correct option is 6.
What is the number of possible spectral lines if electron in 3 excited state of H atom?
Therefore, the correct answer is option [C] 6.
Spectral series of Hydrogen atom
Images related to the topicSpectral series of Hydrogen atom
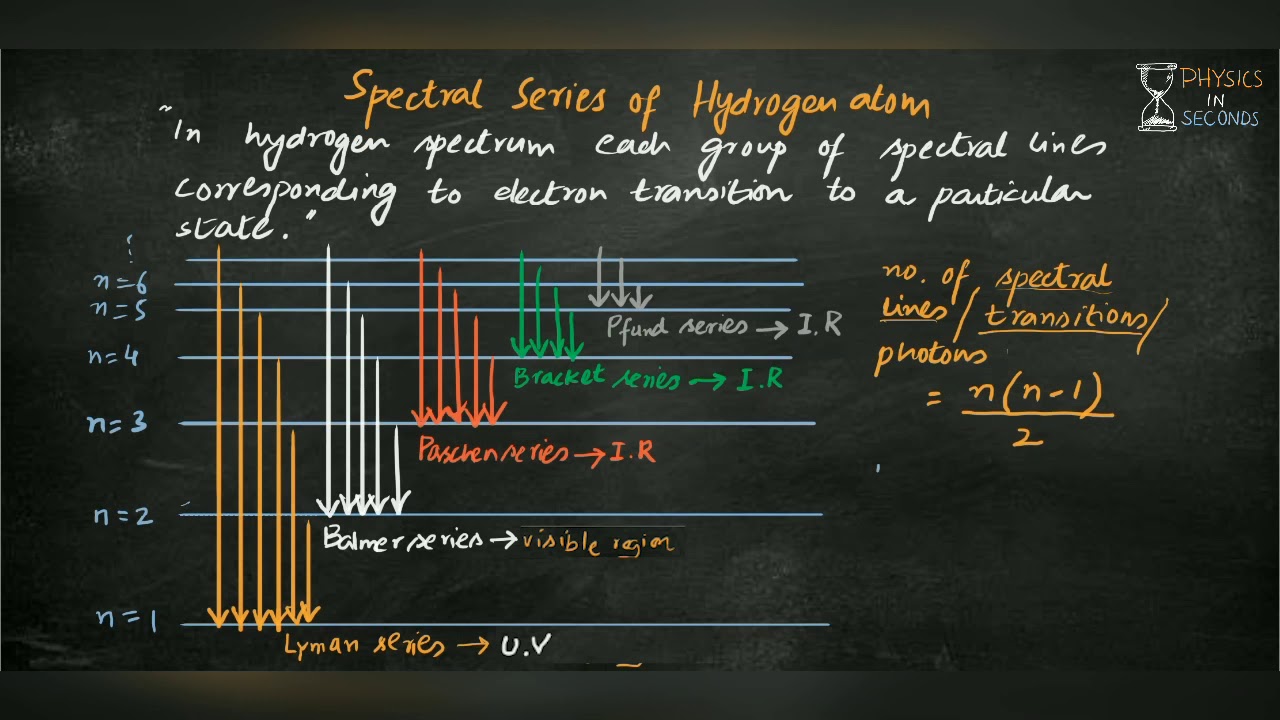
Why does hydrogen only have 4 spectral lines?
Though a hydrogen atom has only one electron, it contains a large number of shells, so when this single electron jumps from one shell to another, a photon is emitted, and the energy difference of the shells causes different wavelengths to be released… hence, mono-electronic hydrogen has many spectral lines.
How many spectral lines are in the Balmer series?
The Balmer equation predicts the four visible spectral lines of hydrogen with high accuracy.
Related searches to How do you find the number of spectral lines in hydrogen spectrum?
- frequency of spectral lines formula
- how many spectral lines does oxygen have
- spectral lines wavelength
- spectral lines of hydrogen
- balmer series
- energy of spectral lines formula
- what determines the number of spectral lines
- emission spectrum
Information related to the topic How do you find the number of spectral lines in hydrogen spectrum?
Here are the search results of the thread How do you find the number of spectral lines in hydrogen spectrum? from Bing. You can read more if you want.
You have just come across an article on the topic How do you find the number of spectral lines in hydrogen spectrum?. If you found this article useful, please share it. Thank you very much.