Are you looking for an answer to the topic “How is Dalton’s law used in real-life?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
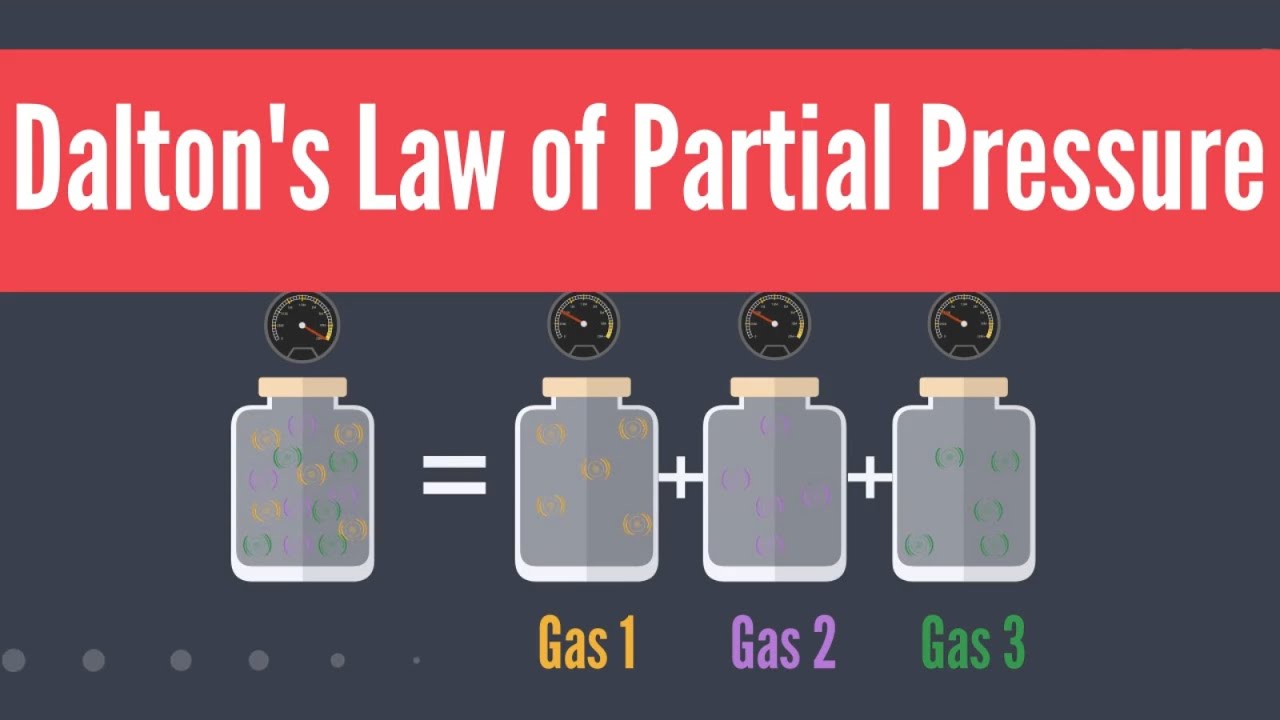
People who ascend to high altitudes experience Dalton’s law when they try to breathe. As they climb higher, oxygen’s partial pressure decreases as total atmospheric pressure decreases in accordance with Dalton’s law. Oxygen has a difficult time making it into the bloodstream when the gas’s partial pressure decreases.For example, the total pressure exerted by a mixture of two gases A and B is equal to the sum of the individual partial pressures exerted by gas A and gas B (as illustrated below).Because each gas in a mixture is at the same temperature and is contained in the same volume (i.e. E-cylinder) Dalton’s law states that their individual partial pressures can be simply added to find the total pressure in a container.

Table of Contents
What is an example of Dalton’s law?
For example, the total pressure exerted by a mixture of two gases A and B is equal to the sum of the individual partial pressures exerted by gas A and gas B (as illustrated below).
Where is Dalton’s law applied?
Because each gas in a mixture is at the same temperature and is contained in the same volume (i.e. E-cylinder) Dalton’s law states that their individual partial pressures can be simply added to find the total pressure in a container.
Dalton’s Law of Partial Pressures Demonstration
Images related to the topicDalton’s Law of Partial Pressures Demonstration

What are some real life applications of ideal gas law?
Ideal gas laws are used for the working of airbags in vehicles. When airbags are deployed, they are quickly filled with different gases that inflate them. The airbags are filled with nitrogen gases as they inflate. Through a reaction with a substance known as sodium azide, the nitrogen gas is produced.
What are other real life applications of this law or other gas laws that you have learned?
You can observe a real-life application of Boyle’s Law when you fill your bike tires with air. When you pump air into a tire, the gas molecules inside the tire get compressed and packed closer together. This increases the pressure of the gas, and it starts to push against the walls of the tire.
What is a real life example of Charles Law?
Tyres of untouched vehicles get deflated during freezing winter days while get inflated in hot summer days. This unusual behaviour is because of Charles’s law. In winter due to low temperatures, the air inside a tyre gets cooler, and they shrink. While in hot days, the air expands with temperature.
What are the application of Dalton’s Law of partial pressure?
Application of Dalton’s Law of Partial pressure of gas-
The pressure of gases over the surface of the liquid can be calculated by using Dalton’s Law of partial pressure. The total pressure of a mixture of gas and water will be equal to atmospheric pressure if the water level inside and outside the vessel are equal.
Why is Dalton’s law important?
This law explains that the sum of all partial pressures of gas in a mixture should equal the barometric pressure. It is an especially important calculation for oxygen to determine the pressure of gas pushing into the alveoli and thus into the circulating blood.
See some more details on the topic How is Dalton’s law used in real-life? here:
Modern Uses and application – Dalton’s Law – Google Sites
Dalton’s law refers to the effects of which partial pressure might have on scuba divers. While the total gas pressure increases as a diver increases their …
What is the application of Dalton’s law of partial pressure?
Jet aeroplanes flying at high altitude need pressurization of cabin such that the partial pressure of oxygen is sufficient for breathing, as the air pressure …
Daltons Law Real Life Examples
Dalton’s Law, simply stated, is that the total pressure exerted by a mixture of gases (real or ideal) in a fixed volume is equal to the sum of each gases …
Real-life applications – Gases – Introduction to the Gas Laws
Applications of Dalton’s and Henry’s Laws · PARTIAL PRESSURE: A MATTER OF LIFE AND POSSIBLE DEATH FOR SCUBA DIVERS. · OPENING A SODA CAN. · FIRE EXTINGUISHERS.
How is Dalton’s law applied to anesthesia?
DALTON’S LAW OF PARTIAL PRESSURES
Inhaled anesthetics will diffuse from the lungs to the blood until the partial pressures in the alveoli and blood are equal.
Daltons Law of Partial Pressure
Images related to the topicDaltons Law of Partial Pressure
Is Dalton’s law applicable to real gases?
Real Gases
Therefore at high pressures and low temperatures, Dalton’s law is not applicable since the gases are more likely to react and change the pressure of the system.
How does the ideal gas law relate to hot air balloons?
The heated air, the air that is inside the balloon, is less dense than the cool air, the air outside of the balloon. And objects that are less dense rise. The reason we know that the hot air is less dense than cool air is due to the Ideal Gas Law.
What gas laws apply to airbags?
Vehicle airbags work using the ideal gas law. By reacting Sodium Azide, , with excess heat, a large amount of Nitrogen gas () is created.
How is baking related to Charles Law?
Bread and delicious cakes are also gifts of Charles’ law. In bakery products yeast is used for fermentation. Yeast produces CO2 and when we bake bread/ cake CO2 expands due to increasing temperature and gives fluffiness to our bread and cakes.
Is a hot air balloon An example of Charles Law?
The law that explains how hot air balloons work is the Charles’s Law. Explanation: if gas expands when it is heated, a given weight of hot air occupies a larger volume then the same weight of cold air. Hot air is less dense than cold air.
How is Charles law apply in hot air balloons?
Charles’s Law says that the volume of a gas is directly related to the temperature of that gas, similarly when a gas is heated, like a burner in a hot air balloon, the gas expands. So when the air inside the balloon expands, it becomes less dense and provides the lift for the hot air balloon.
Why hot air balloon is an example of Charles Law?
As a result of his work with balloons, Charles noticed that the volume of a gas is directly proportional to its temperature. This relationship between the temperature and volume of a gas, which became known as Charles’ law, provides an explanation of how hot-air balloons work.
THE GAS LAWS IN REAL LIFE
Images related to the topicTHE GAS LAWS IN REAL LIFE

How does Dalton’s law relate to breathing?
Dalton’s Law in Respiration
Dalton’s law states that at any given time, the percentage of each of these gasses in the air we breathe makes its contribution to total atmospheric pressure, and this contribution will depend on how much of each gas is in the air we breathe.
What is partial pressure and why is it important?
Partial pressure is the force exerted by a gas. The sum of the partial pressures of all the gases in a mixture equals the total pressure. Partial pressure is extremely important in predicting the movement of gases. Recall that gases tend to equalize their pressure in two regions that are connected.
Related searches to How is Dalton’s law used in real-life?
- examples of gas laws in real life
- how is dalton’s law used in real life quizlet
- avogadro’s gas law application in real life
- what is the importance of gas laws in our daily life
- how is dalton’s law used in real life in real life
- how is dalton’s law used in real life today
- application of dalton’s law of partial pressure
- application of daltons law of partial pressure
- examples of ideal gas laws in everyday life
- boyles law application in real life
- combined gas law application in real life
- boyle’s law application in real life
- avogadros gas law application in real life
- applications of gases in daily life
Information related to the topic How is Dalton’s law used in real-life?
Here are the search results of the thread How is Dalton’s law used in real-life? from Bing. You can read more if you want.
You have just come across an article on the topic How is Dalton’s law used in real-life?. If you found this article useful, please share it. Thank you very much.