Are you looking for an answer to the topic “How is de Broglie wavelength related to height?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Hence, the de-Broglie wavelength of the particle as a function of height is proportional to \[{H^{\dfrac{{ – 1}}{2}}}\] .The De Broglie wavelength doesn’t directly depend on the charge; however, it depends on the momentum. Therefore, if an electric field accelerates a charged particle, then the momentum acquired would depend on the charge.λαH−1/2.
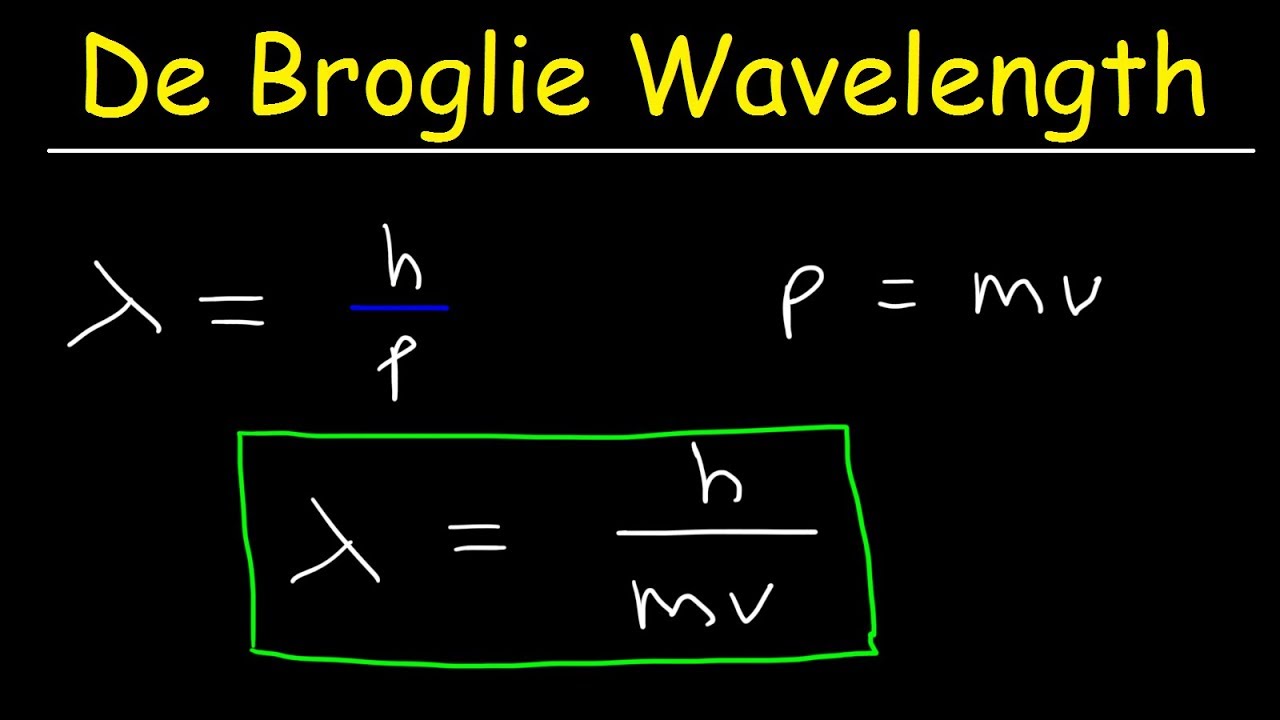
Table of Contents
What does the de Broglie wavelength depend on?
The De Broglie wavelength doesn’t directly depend on the charge; however, it depends on the momentum. Therefore, if an electric field accelerates a charged particle, then the momentum acquired would depend on the charge.
Is dropped from a height h the de Broglie wavelength of the particle as a function of height is proportional to?
λαH−1/2.
De Broglie Wavelength Problems In Chemistry
Images related to the topicDe Broglie Wavelength Problems In Chemistry

How is de Broglie wavelength related to velocity?
According to de Broglie, The relation between the wavelength of the wave associated with the moving electron and momentum of the electron is λ=mvh.
What is the relation between de Broglie wavelength and potential?
The De Broglie wavelength is inversely proportional to the square root of the potential.
What is de Broglie equation explain relation between wavelength and momentum with the help of de Broglie equation?
λ = h m v = h momentum : where ‘h’ is the Plank’s constant. This equation relating the momentum of a particle with its wavelength is de Broglie equation and the wavelength calculated using this relation is de Broglie wavelength.
What is de Broglie’s relation?
De Broglie proposed that as light exhibits both wave-like and particle-like properties, matter to exhibit wave-like and particle-like properties. This nature was described as dual behaviour of matter. On the basis of his observations, de Broglie derived a relationship between wavelength and momentum of matter.
What is the de Broglie wavelength of a ball of mass 200 g?
answer : option (d) 10^-30 m.
See some more details on the topic How is de Broglie wavelength related to height? here:
What is the de Broglie length? – Neighborshateus.com
The de-broglie wavelength of the particle as a funciton of height is proportional to.
Question 11.1, 11. DUAL NATURE OF RADIATION AND …
Therefore, de-Broglie wavelength is inversely proportional to the square root of Height. Option ( …
A particle is dropped from a height H. The de – Infinity Learn
A particle is dropped from a height H. The de – Broglie wavelength associated with particle is proportional to · An electron is orbiting in 4 energy state in a …
How the wavelength of moving particles are related to the momentum of electron?
The wavelength of a matter wave associated with a particle is inversely proportional to the magnitude of the particle’s linear momentum.
When a particles Ke increases what happen to its de Broglie wavelength?
When a particle’s kinetic energy increases, what happens to its de Broglie wavelength? As kinetic energy increases, so does momentum. Momentum and wavelength are inversely related to one another (lambda = h / p), so the wavelength must decrease.
How does the de Broglie wavelength of an electron change if its momentum increases?
How does the de Broglie wavelength of an electron change if its momentum increases? How does the de Broglie wavelength of an electron change if its kinetic energy decreases? The de Broglie wavelength of the electron increases.
De Broglie wavelength | Physics | Khan Academy
Images related to the topicDe Broglie wavelength | Physics | Khan Academy

What is the relation between de Broglie wavelength and temperature?
λD = λth = thermal de Broglie wavelength of the gas particles.
What is the relationship between wavelength and voltage?
There is a simple relationship between wavelength, frequency, and velocity. You can see this easily: a wave travels one wavelength L in one period P, so v = L/P. The period P is defined as the inverse of the frequency f, so P = 1/f. Put these together and you get v = Lf.
What is the relation between lambda and potential difference?
The de Broglie wavelength λ=1.23 x 10−9m . If a charge of 1 Coulomb is moved through a potential difference of 1 Volt then 1 Joule of work is done. This is how the Volt is defined. So if a charge of e coulombs is moved through a potential difference of V volts then the work done is eV Joules.
How is the momentum of a particle related with its de Broglie wavelength show the variation on a graph?
By this relation we can conclude that the linear momentum of a photon is inversely proportional to the de Broglie wavelength. The graph of p vs λ shall be a rectangular hyperbola.
What is the relation between momentum and the wavelength of a photon?
The momentum of a photon is directly proportional to its frequency and inversely proportional to its wavelength. If the wavelength of a photon is known, its momentum can be calculated using the formula 𝑝 = ℎ 𝜆 .
What is the main point of the de Broglie equation?
The main point of de Broglie’s equation is that particles can behave like waves. Classical physics defined waves and particles as two separate physical phenomenons, but de Broglie proposed that matter could behave like waves and derived his equation based on that idea.
What is the significance of de Broglie’s wave equation Brainly?
Answer: The de Broglie equation relates a moving particle’s wavelength with its momentum. The de Broglie wavelength is the wavelength, λ, associated with a massive particle and is related to its momentum, p, through the Planck constant, h: In other words, you can say that matter also behaves like waves.
What is de Broglie wavelength associated with a ball of mass 100g moving with a speed of 300m S?
Answer: The wavelength associated with the ball is 66 m.
The de Broglie Wavelength and Wave Particle Duality – A Level Physics
Images related to the topicThe de Broglie Wavelength and Wave Particle Duality – A Level Physics
What will be the wavelength of a ball of mass 200 gram and moving with a velocity hundred Metre per second?
λ=2. 385×10−30m.
What will be the wavelength of a ball of mass 0.2 kg moving with velocity of 10 ms?
A ball of mass 0.2 is moving with a velocity of 10 ms^–1. Its wavelength will be (Planck constant = 6.626 × 10^–34 Js.) A ball of mass 0.2 is moving with a velocity of 10 ms–1. Its wavelength will be (Planck constant = 6.626 × 10–34 Js.)
Related searches to How is de Broglie wavelength related to height?
- how is de broglie wavelength related to height and weight
- how is de broglie wavelength related to height of electrons
- how is de broglie wavelength related to height of a wave
- how is de broglie wavelength related to height and mass
- how is de broglie wavelength related to height and width
Information related to the topic How is de Broglie wavelength related to height?
Here are the search results of the thread How is de Broglie wavelength related to height? from Bing. You can read more if you want.
You have just come across an article on the topic How is de Broglie wavelength related to height?. If you found this article useful, please share it. Thank you very much.