Are you looking for an answer to the topic “How is extraction emulsion prevented?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
The simplest way to prevent the formation of an emulsion is to gently swirl instead of shake the separatory funnel. By swirling the separatory funnel the agitation that can cause the emulsion to form is reduced, but the surface area of contact between the two phases is maintained to allow for extraction to occur.The formation of an emulsion is a common problem when performing extractions. An emulsion is a stable dispersion of one liquid in a second immiscible liquid. Emulsions delay the separation of two liquids, making it necessary to “break” the emulsion.Occasionally, emulsions can form while the liquids are being mixed in the separatory funnel. An emulsion occurs when small droplets of an organic solvent are suspended in a water solution. Emulsions can be difficult to disperse, however, a few techniques may be attempted.
- Multiplication of extraction steps to obtain optimum output.
- Use of large volumes of organic solvents, which the costs of recycling are becoming increasingly more expensive.
- Emulsion’s difficulties which hinders the full recovery of the extract.
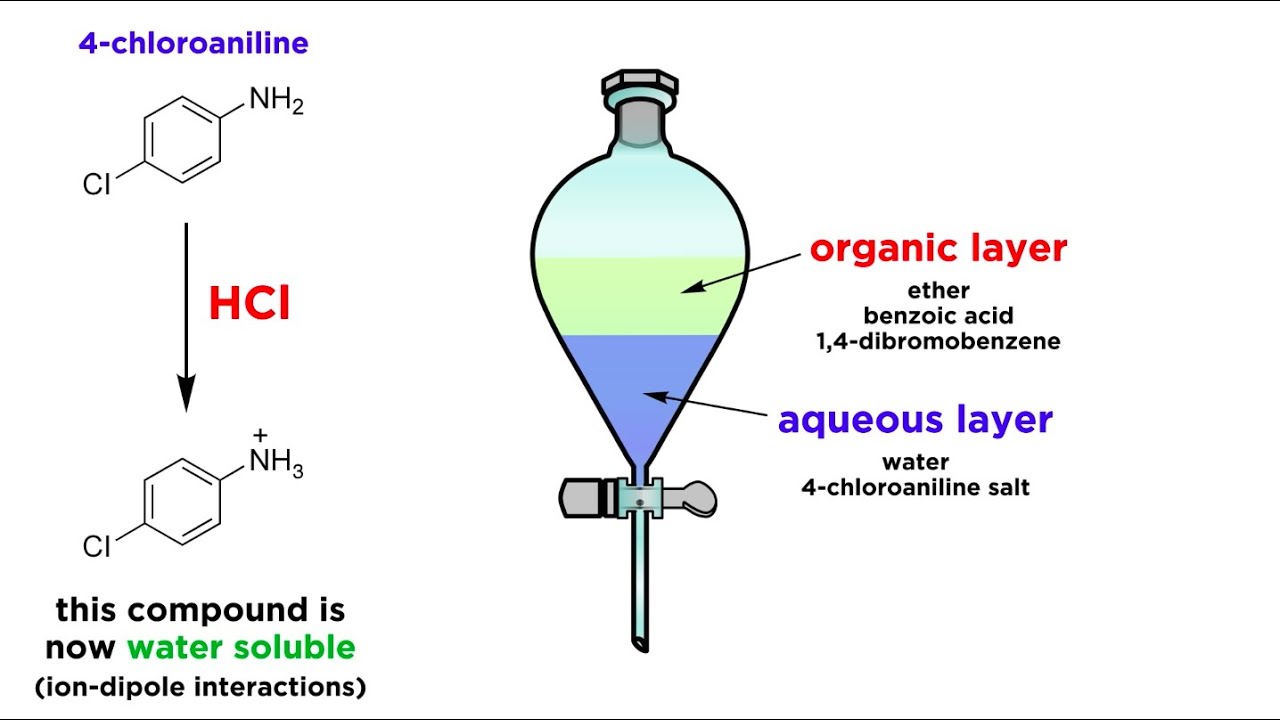
Table of Contents
How does emulsion affect extraction?
The formation of an emulsion is a common problem when performing extractions. An emulsion is a stable dispersion of one liquid in a second immiscible liquid. Emulsions delay the separation of two liquids, making it necessary to “break” the emulsion.
Why do emulsions form during extractions?
Occasionally, emulsions can form while the liquids are being mixed in the separatory funnel. An emulsion occurs when small droplets of an organic solvent are suspended in a water solution. Emulsions can be difficult to disperse, however, a few techniques may be attempted.
Separating Components of a Mixture by Extraction
Images related to the topicSeparating Components of a Mixture by Extraction

What factors cause an emulsion to break?
How do emulsions break? Emulsions are destabilized by four different mechanisms: creaming/sedimentation, flocculation, coalescence, and Ostwald ripening. Creaming occurs when the emulsion separates due to a density difference where the lighter oil droplets rise to the surface.
What are the disadvantages of liquid liquid extraction?
- Multiplication of extraction steps to obtain optimum output.
- Use of large volumes of organic solvents, which the costs of recycling are becoming increasingly more expensive.
- Emulsion’s difficulties which hinders the full recovery of the extract.
How can an emulsion be eliminated?
Emulsions can be disrupted by the addition of brine or salt water, which increases the ionic strength of the aqueous layer and facilitates separation of the two phases by forcing the surfactant-like molecule to separate into one phase or the other-this technique is known as salting out.
How are emulsions minimized?
Filtration – sometimes filters are effective at removing the emulsion or separating the aqueous layer from the organic layer. Glass wool can be used as a physical barrier to catch the emulsion. The phase separation layer can be used to separate the aqueous layer from the organic layer (or vice versa).
What is emulsion in solvent extraction?
Certain types of samples, such as those containing detergent, may form emulsions when doing an oil in water extraction into a solvent. The boundary between the solvent and the sample will have an emulsion layer that has a cloudy or milky appearance as shown in this photo.
See some more details on the topic How is extraction emulsion prevented? here:
Tackling Emulsions Just Got Easier – Sample Prep
How do you prevent the formation of an emulsion? Process your samples using solid-phase extraction (SPE) instead of liquid-liquid extraction.
How is extraction emulsion prevented? – Cement Answers
How is extraction emulsion prevented? The simplest way to prevent the formation of an emulsion is to gently swirl instead of shake the separatory funnel.
Emulsion Problem Encountered in Extractions – BrainKart
It may be defined as-‘a dispersed system containing at least two immiscible liquid phases’. The effective and meaningful extraction of an …
Emulsion Breaking in Water Solvent Extractions – AZoM
If the emulsion occurs due to a surfactant, detergent or alkali soap, then reducing the pH to 2 with hydrochloric acid (HCl), or sulfuric acid ( …
What causes emulsion?
An emulsion is formed when two nonsoluble liquids (e.g., an oil and water) are agitated together to disperse one liquid into the other, in the form of drops. Emulsions can either be oil-in-water (O/W) or water-in-oil (W/O), depending on whether the continuous phase is the water or the oil, respectively.
Why is sodium bicarbonate used in extraction?
This phenomenon will often be observed if sodium bicarbonate is used for the extraction in order to neutralize or remove acidic compounds. The reaction affords carbon dioxide (CO2), which is a gas at ambient temperature. Pressure builds up that pushes some of the gas and the liquid out.
What is an Emulsion?
Images related to the topicWhat is an Emulsion?
How do you prevent phase separation in emulsion?
To prevent phase separation in cases where the phase densities cannot be changed, it is common to increase the viscosity of the emulsion – either by dissolving thickeners in the continuous phase or formulating the emulsion with a high volume fraction of dispersed phase so that droplet packing itself gives rise to …
How can you increase the stability of an emulsion?
To increase the kinetic stability of emulsions, stabilisers such as emulsifiers, weighting agents, ripening inhibitors and texture modifiers (thickeners and gelling agents) are often used [16].
Which instability step is prevented by emulsifier?
…
| Q. | In the stability of emulsion, which instability step is prevented by emulsifiers?: |
|---|---|
| D. | flocculation |
| Answer» b. coalescence |
How can extraction efficiency be improved?
- The Crush. Finely crushed malt yields more extract. …
- Temperature. There are two temperature-related effects on extract efficiency. …
- Stirring. Stirred mashes yield more extract. …
- Sparge Volume. …
- Sparging Time. …
- Suggestions.
What are the factors affecting solvent extraction?
- EFFECT OF TEMPERATURE AND INERT SOLUTES. The physical as well as chemical interactions of a solute is capable of changing its apparent partition coefficient between a pair of solvents. …
- EFFECT OF pH ON EXTRACTION. …
- EFFECT OF ION-PAIR FORMATION. …
- EFFECT OF SYNERGISTIC EXTRACTION.
What are the limitations of solvent extraction?
Disadvantages of solvent extraction are, first, that the solvent will also dissolve unwanted pyrolysis products, matrix materials, and other substances, some of which may interfere with the subsequent analysis and second, that evaporation of the solvent may also cause evaporation of some of the volatile components of …
What are instabilities of emulsion?
There are three types of instability in emulsions: (1) flocculation, (2) creaming, and (3) coalescence. Flocculation occurs when there is an attractive force between the droplets, so they form flocs.
Why is dichloromethane a good solvent for extraction?
While dichloromethane isn’t miscible with water, it is able to dissolve a wide range of organic compounds. These properties, combined with its volatility, makes DCM a highly effective solvent in many industrial processes.
What are Emulsions? | Properties of Matter | Chemistry | FuseSchool
Images related to the topicWhat are Emulsions? | Properties of Matter | Chemistry | FuseSchool

What is creaming and cracking of emulsion?
Creaming occur when isolated oil droplets combine and rise to the top of an oil water emulsion or sink to the bottom in w/o emulsions. In both scenarios, the emulsion can be easily re-dispersed by shaking. Coalescence (breaking or cracking) is the total and irreversible division and fusion of the dispersed stage.
What is the emulsion process?
Abstract. Emulsification is the process of dispersing two or more immiscible liquids together to form a semistable mixture. In food applications, these two liquids generally consist of an organic (oil) phase and an aqueous (water) phase that is stabilized by the addition of a food-grade emulsifier (surfactant).
Related searches to How is extraction emulsion prevented?
- how is extraction emulsion prevented by
- how to break an emulsion in a liquid-liquid extraction
- liquid liquid extraction brine
- liquid liquid extraction discussion
- salting out emulsion
- how to break an emulsion in a liquid liquid extraction
- sources of error in liquid liquid extraction
- liquid-liquid extraction brine
- why is liquid liquid extraction important
- which type of solvents are more prone to developing emulsions?
- how is extraction emulsion prevented from drying out
- why do emulsions form during extractions
- how is extraction emulsion prevented from melting
- how is extraction emulsion prevented from working
- why is liquid-liquid extraction important
- which type of solvents are more prone to developing emulsions
- how is extraction emulsion prevented from curing
- how is liquid liquid extraction different from acid base extraction
Information related to the topic How is extraction emulsion prevented?
Here are the search results of the thread How is extraction emulsion prevented? from Bing. You can read more if you want.
You have just come across an article on the topic How is extraction emulsion prevented?. If you found this article useful, please share it. Thank you very much.