Are you looking for an answer to the topic “How much heat does it take to get 100g of 100 C water to evaporate?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
The specific heat of vaporization of water is 2230 J/g, so evaporating 100g of water will take 223,000 J.Water’s heat of vaporization is around 540 cal/g at 100 °C, water’s boiling point.This means that to convert 1 g of water at 100 ºC to 1 g of steam at 100 ºC, 2260 J of heat must be absorbed by the water. Conversely, when 1 g of steam at 100 ºC condenses to give 1 g of water at 100 ºC, 2260 J of heat will be released to the surroundings.

Table of Contents
What is the heat of vaporization of water at 100 C?
Water’s heat of vaporization is around 540 cal/g at 100 °C, water’s boiling point.
How much heat energy must be transferred away from 100 g of steam at 100 C to change it completely to a liquid?
This means that to convert 1 g of water at 100 ºC to 1 g of steam at 100 ºC, 2260 J of heat must be absorbed by the water. Conversely, when 1 g of steam at 100 ºC condenses to give 1 g of water at 100 ºC, 2260 J of heat will be released to the surroundings.
Specific Heat Capacity Problems Calculations – Chemistry Tutorial – Calorimetry
Images related to the topicSpecific Heat Capacity Problems Calculations – Chemistry Tutorial – Calorimetry

How much energy does it take to evaporate 1g of water?
energy known as the latent heat of vaporization is required to break the hydrogen bonds. At 100 °C, 540 calories per gram of water are needed to convert one gram of liquid water to one gram of water vapour under normal pressure.
What is the enthalpy of evaporation of water?
| Compound | Boiling point, at normal pressure | Heat of vaporization |
|---|---|---|
| (K) | (J/mol) | |
| Propane | 231 | 15700 |
| Phosphine | 185 | 14600 |
| Water | 373.15 | 40660 |
How much heat must be added to 900 grams of water at 100 C to make it steam at 100 C?
Converting liquid water to steam at 100°C (0.101325 MPa) requires a heat addition of 539 cal/g (2260 J/gm).
How do I calculate heat?
We wish to determine the value of Q – the quantity of heat. To do so, we would use the equation Q = m•C•ΔT. The m and the C are known; the ΔT can be determined from the initial and final temperature.
How much heat is given up when 20g of steam at 100 C is condensed and cooled to 20 C?
Heat change = Condensation heat change )+( Heat change of water during cooling) =mLv+cmΔT=(20 g)(−540cal/g)+(1.00cal/g⋅∘C)(20 g)(20∘C−100∘C)=−12400cal=−12kcal=−50 kJ.
See some more details on the topic How much heat does it take to get 100g of 100 C water to evaporate? here:
Heat of Vaporization
For water at its normal boiling point of 100 ºC, the heat of vaporization is 2260 J g-1. This means that to convert 1 g of water at 100 ºC to 1 g of steam at …
How much energy is needed to vaporize a gram of water?
The specific heat of vaporization of water is 2230 J/g, so evaporating 100g of water will take 223,000 J.
How much heat is required to vaporize 80.6 g of water at 100 …
Now, multiply moles by the heat of vaporization, 40.7 kJ / mol e and you should get your answer.
How much heat is required to melt 100g ice? – AnswersToAll
In this case, we have 100g of water at 100C and we …
How much energy does it take to vaporize 1 kg of water?
After some research, I found that it takes approx. 2.3 Megajoules to evaporate 1 kilogram of water which is @ room temp. Since the mass of room temp water = approx. 1Kg, this means it would take approx. 7,500 watts-hours to completely evaporate 1 liter of water in 5 minutes.
How much energy does it take to vaporize?
According to the captured study, it takes around three gigajoules of death-ray to entirely vaporize a person—enough to completely melt 5,000 pounds of steel or simulate a lightning bolt.
How many joules of energy are needed to change the temperature of 100.0 grams of water from 20.0 C to 40.0 C?
1 Answer. Ernest Z. To convert 100.0 g of water at 20.0 °C to steam at 100.0 °C requires 259.5 kJ of energy.
Heating Curve and Cooling Curve of Water – Enthalpy of Fusion Vaporization
Images related to the topicHeating Curve and Cooling Curve of Water – Enthalpy of Fusion Vaporization
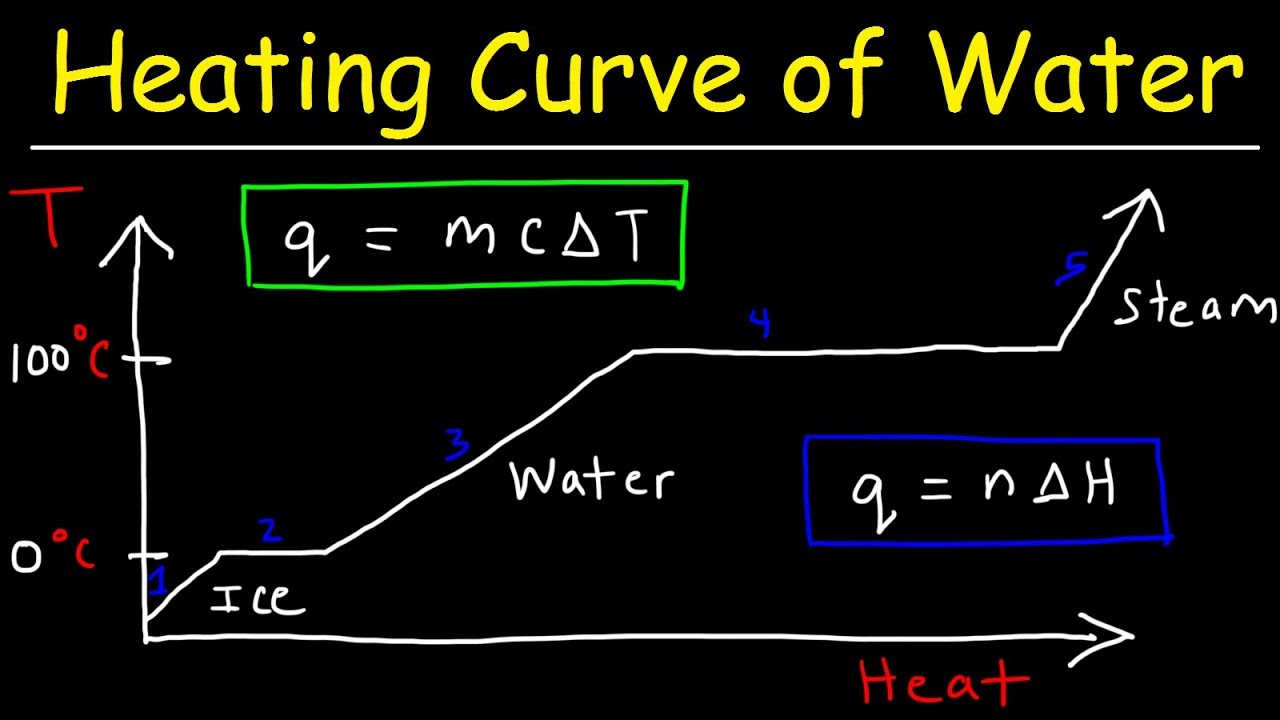
How do you find heat of vaporization from boiling point?
…
[edit] Using Riedel’s equation.
| where: | |
|---|---|
| Hv | = Heat of vaporization, in J/mol |
| R | = 8.3144 = Universal gas constant, in J/(K mol) |
| Tn | = The liquid’s normal boiling point, in K |
| Tc | = The liquid’s critical temperature, in K |
How do you find heat of vaporization given temperature and pressure?
If the problem provides the two pressure and two temperature values, use the equation ln(P1/P2)=(Hvap/R)(T1-T2/T1xT2), where P1 and P2 are the pressure values; Hvap is the molar heat of vaporization; R is the gas constant; and T1 and T2 are the temperature values.
What is the quantity of heat required to raise the temperature of 1 gram of water through 1 C?
For example, the specific heat of water is 1 calorie (or 4.186 joules) per gram per Celsius degree.
How do you calculate evaporation rate?
- gh=ΘA(xs−x)
- gh = amount of evaporated water per hour (kg/h)
- Θ=(25+19v) = evaporation coefficient (kg/(m2 h))
- v = velocity of air above the water surface (m/s)
- A = water surface area (m2)
How do you calculate water enthalpy?
- Formula. H = m * C * T.
- Mass (g)
- Specific Heat (J/g*C)
- Temperature (C)
How do you vaporize water?
TL;DR: When trying to make water evaporate quickly, it is best to spread the water over a large surface area and apply heat as evenly as possible. If using hot air to evaporate water, increased velocity will increase the speed of evaporation.
At what temp does water evaporate?
Energy is used to break the bonds that hold water molecules together, which is why water easily evaporates at the boiling point (212° F, 100° C) but evaporates much more slowly at the freezing point. Net evaporation occurs when the rate of evaporation exceeds the rate of condensation.
How do you calculate the heat required to raise the temperature?
The specific heat capacity of a substance is the quantity of heat needed to raise the temperature of a unit quantity of the substance by one degree. Calling the amount of heat added Q, which will cause a change in temperature ∆T to a weight of substance W, at a specific heat of material Cp, then Q = w x Cp x ∆T.
How much heat is required to convert 12gms of ice at -10c to water vapour at 100c?
Images related to the topicHow much heat is required to convert 12gms of ice at -10c to water vapour at 100c?

What will be the heat released by converting 1 kg of steam at 150 C into 1 kg of water at 50 C?
Solution : `H = 1 xx (1)/(2) xx 50 +1 xx 540 +1 xx1 xx 50` <br> `= 540 +75 = 615 Kcal` <br> Heat release `= 615 Kcal`. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
What is the Calorimetry equation?
Formula Related to Calorimetry
The basic concept of calorimetry is as follows. The heat released by the hot object = Heat absorbed by the cold object. The transfer of heat is evaluated with the help of a formula, which is as follows. Q = mCΔT.
Related searches to How much heat does it take to get 100g of 100 C water to evaporate?
- how can water vapor become ice
- how much energy is required to vaporize 10 00 grams of water at its boiling point
- heat of vaporization of water kj/kg
- how can water vapor become ice?
- how much heat is required to vaporize 25 g of water at 100c
- how much heat is required to vaporize 25 g of water at 100˚c?
- what is the total number of kilojoules required to boil 100 grams of water at 100c and 1 atmosphere
- how much energy is released when 1 gram of steam condenses to water at 100 c
- energy required to convert water to steam
- heat of vaporization of water kjkg
- how much heat would be required to heat 10 grams of liquid water from 50 ºc to vapor at 100 ºc?
- heat of vaporization of water
Information related to the topic How much heat does it take to get 100g of 100 C water to evaporate?
Here are the search results of the thread How much heat does it take to get 100g of 100 C water to evaporate? from Bing. You can read more if you want.
You have just come across an article on the topic How much heat does it take to get 100g of 100 C water to evaporate?. If you found this article useful, please share it. Thank you very much.