Are you looking for an answer to the topic “How much heat is released by the neutralization reaction in kJ?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Enthalpy changes of neutralization are always negative – heat is released when an acid and and alkali react. For reactions involving strong acids and alkalis, the values are always very closely similar, with values between -57 and -58 kJ mol–1.Enthalpy of neutralization is the heat evolved when one gram equivalent of the acid is completely neutralized by a base in dilute solution. The chemical reaction is given below. 13.7 kcal of heat is liberated out and is the heat of neutralization for all strong acids and bases.SInce strong acids and strong bases are completely dissociated in solution, no formal bonds are being broken. The formation of two very strong covalent bonds between hydrogen and the hydroxide ion is responsible for the neutralization reaction’s exothermic character.
- Step 1: Calculate the heat evolved: q = m × Cg × ΔT. …
- Step 2: Calculate the enthalpy change for the reaction: ΔH = −q.
- Step 3: Calculate the molar enthalpy of neutralisation: ΔneutH = ΔH ÷ n(H2O(l))
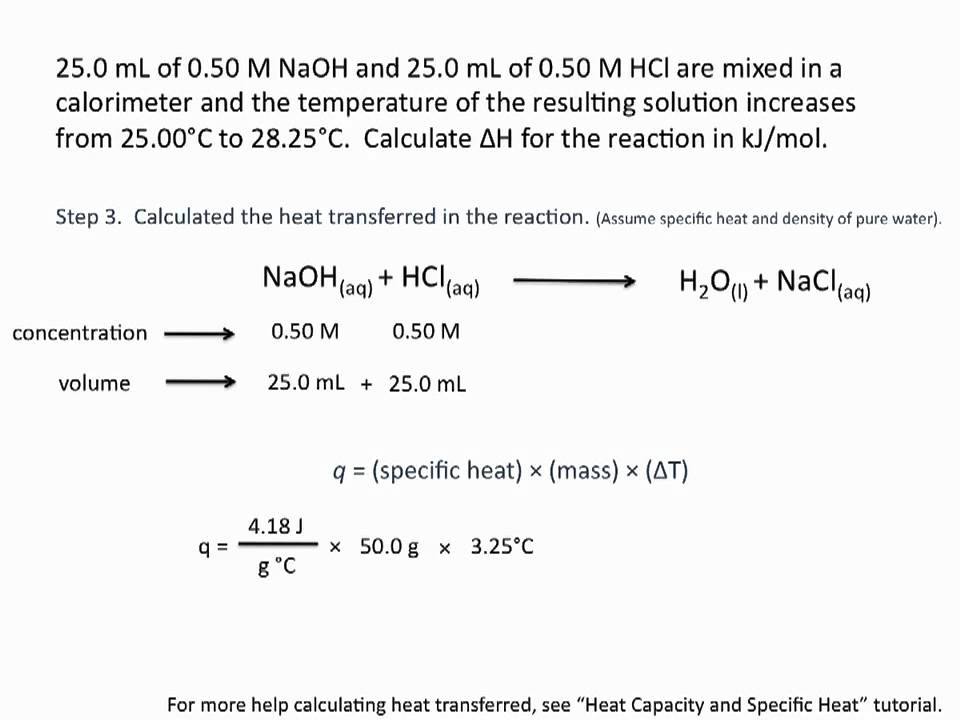
Table of Contents
How much heat is released by the neutralization reaction?
Enthalpy of neutralization is the heat evolved when one gram equivalent of the acid is completely neutralized by a base in dilute solution. The chemical reaction is given below. 13.7 kcal of heat is liberated out and is the heat of neutralization for all strong acids and bases.
How do you calculate the enthalpy of a neutralization reaction?
- Step 1: Calculate the heat evolved: q = m × Cg × ΔT. …
- Step 2: Calculate the enthalpy change for the reaction: ΔH = −q.
- Step 3: Calculate the molar enthalpy of neutralisation: ΔneutH = ΔH ÷ n(H2O(l))
Using Calorimetry to Calculate Enthalpies of Reaction – Chemistry Tutorial
Images related to the topicUsing Calorimetry to Calculate Enthalpies of Reaction – Chemistry Tutorial

Why is heat released in a neutralization reaction?
SInce strong acids and strong bases are completely dissociated in solution, no formal bonds are being broken. The formation of two very strong covalent bonds between hydrogen and the hydroxide ion is responsible for the neutralization reaction’s exothermic character.
What is the heat of neutralization of HCl and NaOH?
The heat of neutralization of HCl by NaOH is -55.9 kJ/mol.
What is molar heat of neutralization?
Explanation: Molar heat of neutralisation: the energy liberated per mole during a neutralisation reaction. Energy liberated for 5 mol is 13793 J. For 1 mol. ΔHneu=13793 J5 mol=2758.6 J/mol=2.7586 kJ/mol.
What is enthalpy of neutralization with example?
The enthalpy of neutralization (ΔHn) is the change in enthalpy that occurs when one equivalent of an acid and a base undergo a neutralization reaction to form water and a salt. It is a special case of the enthalpy of reaction. It is defined as the energy released with the formation of 1 mole of water.
Enthalpy Stoichiometry Part 2: How to Find Heat Released
Images related to the topicEnthalpy Stoichiometry Part 2: How to Find Heat Released

See some more details on the topic How much heat is released by the neutralization reaction in kJ? here:
How to Calculate the Molar Heat of Neutralization – Sciencing
Calculate the number of moles of base you add to determine the molar heat of neutralization, expressed using the equation ΔH = Q ÷ n, where “n” …
Enthalpy of Neutralisation Chemistry Tutorial – AUS-e-TUTE
Less than 55.90 kJ mol-1 of energy is released when: … Now we can calculate the heat released by this neutralisation reaction, q,. q = m × Cg × ΔT.
Enthalpy of neutralization – Wikipedia
The heat of ionization for this reaction is equal to (–12 + 57.3) = 45.3 kJ/mol at 25 °C. References …
Heat of Neutralization: HCl(aq) + NaOH(aq) – Chemdemos
HCl(aq) + NaOH(aq) –> NaCl(aq) + H2O(l) + Energy · Thermochemistry determine the heat exchanged at constant pressure, · This reaction is classified as an …
What is the theoretical value of enthalpy of Neutralisation in kJ mol for HCl and NaOH?
The heat of neutralization of HCl by NaOH is -55.9 kJ/mol.
What is the theoretical value of enthalpy of Neutralisation in kJ mol 1 for HCl and NaOH?
Enthalpy of neutralization of HCl by NaOH is – 55.84 kJ/mol and by NH4OH is – 51.34 kJ/mol.
How do you calculate energy released in kJ mol?
…
Worked Example: Calculating Heat Released or Absorbed for a Given Amount of Reactant or Product.
| energy absorbed = | n mol × ΔH kJ mol–1 stoichiometric coefficient |
|---|---|
| energy absorbed = | 0.2498 mol × 198 kJ mol–1 2 |
| = | 24.73 kJ |
| = | 24.7 kJ |
How do you find Delta H from kJ mol?
Subtract the sum of the heats of formation of the reactants from that of the products to determine delta H: delta H = –110.53 kJ/mol – (–285.83 kJ/mol) = 175.3 kJ.
What is the heat of the reaction?
heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the same temperature.
Calculate the amount of heat released when heat of neutralization is `-57.0 kJ :` `(a)`
Images related to the topicCalculate the amount of heat released when heat of neutralization is `-57.0 kJ :` `(a)`

What is the heat of reaction for the neutralization reaction between nh3 and HCl?
The heat of neutralization reaction between hydrochloric acid and ammonia solution is – 53.4 kJ/mol.
Why enthalpy of Neutralisation is always 57.1 kJ for strong acids and strong base?
The enthalpy of neutralization of any strong acid and strong base is always constant, i.e. 57.1 kJ because both the acid and base undergo complete ionization.
Related searches to How much heat is released by the neutralization reaction in kJ?
- heat of neutralization
- heat of neutralization of hcl and naoh lab report
- how to calculate enthalpy of neutralization
- heat of neutralization calculator
- heat of neutralization of hcl and naoh
- how much heat is released by the neutralization reaction in kj
- heat of neutralization lab report pdf
- molar heat of neutralization
- heat of neutralization graph
- in part b how much heat (reaction) is released by the neutralization reaction in kj
Information related to the topic How much heat is released by the neutralization reaction in kJ?
Here are the search results of the thread How much heat is released by the neutralization reaction in kJ? from Bing. You can read more if you want.
You have just come across an article on the topic How much heat is released by the neutralization reaction in kJ?. If you found this article useful, please share it. Thank you very much.