Are you looking for an answer to the topic “Does the value of KC increase/decrease or remain the same when the temperature is decreased?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
If Kc increases with a decreases in temperature, the reaction to shifts to the right. If Kc decreases with a decrease in temperature, the reaction to shifts to the left. If the products dominate in a reaction, the value for K is greater than 1.Changes in Temperature
Kc is larger when the reaction shifts right. This occurs if T is increased for an Endothermic Reaction or T is decreased for an Exothermic reaction. Kc is smaller when the reaction shifts left. This occurs if T is decreased for an Endothermic Reaction or T is increased for an Exothermic reaction.This same idea can be used to understand how temperature will change the value of K. le Châtelier’s principle allows us to predict that if the temperature increases, then the reaction will shift to the left – in other words, the concentration of R will increase, while P will decrease. This means that K will decrease.
…
Changing temperature.
| temperature | Kp |
|---|---|
| 500 K | 160 |
| 700 K | 54 |

Table of Contents
How does KC change when the temperature is decreased?
Changes in Temperature
Kc is larger when the reaction shifts right. This occurs if T is increased for an Endothermic Reaction or T is decreased for an Exothermic reaction. Kc is smaller when the reaction shifts left. This occurs if T is decreased for an Endothermic Reaction or T is increased for an Exothermic reaction.
Does KC increase or decrease when temperature decreases?
…
Changing temperature.
| temperature | Kp |
|---|---|
| 500 K | 160 |
| 700 K | 54 |
Which way will the Equilibrium Shift? (Le Chatelier’s Principle)
Images related to the topicWhich way will the Equilibrium Shift? (Le Chatelier’s Principle)

How does a decrease in temperature affect K?
This same idea can be used to understand how temperature will change the value of K. le Châtelier’s principle allows us to predict that if the temperature increases, then the reaction will shift to the left – in other words, the concentration of R will increase, while P will decrease. This means that K will decrease.
What happens to K value when temperature increases?
Increasing the temperature of a reaction generally speeds up the process (increases the rate) because the rate constant increases according to the Arrhenius Equation. As T increases, the value of the exponential part of the equation becomes less negative thus increasing the value of k.
Does KC depend on temperature?
The numerical value of Kc increases or decreases with temperature and it depends upon the heat of reaction whether it is endothermic or exothermic .
Is KC only affected by temperature?
Equilibrium constants are changed if you change the temperature of the system. Kc or Kp are constant at constant temperature, but they vary as the temperature changes. You can see that as the temperature increases, the value of Kp falls.
Why does increase in temperature decrease KC?
If the reaction is exothermic, increasing the temperature will reduce Kc and vice-verca. This is because if you increase the temp., you drive the equilibrium backwards (in the endothermic direction), and therefore increase the concentration of reactants and decrease the concentration of products.
See some more details on the topic Does the value of KC increase/decrease or remain the same when the temperature is decreased? here:
The Effect of Changing Conditions – Chemistry LibreTexts
If you decrease the concentration of C, the top of the Kc expression gets smaller. That would change the value of Kc.
Why does KC increase when the temperature decreases?
According to Le Chatelier’s principle, decrease in temperature favors exothermic reaction. For a reversible reaction, if the forward reaction is exothermic, …
equilibrium constants and Le Chatelier’s Principle – Chemguide
This is typical of what happens with any equilibrium where the forward reaction is exothermic. Increasing the temperature decreases the value of the equilibrium …
Equilibrium Constants K and Temperature Chemistry Tutorial
If a reaction is exothermic, the value of the equilibrium constant decreases when the reaction mixture is heated. Exothermic reaction: increasing temperature, …
What happens to KC if temperature is decreased in an exothermic reaction?
If Kc increases with a decreases in temperature, the reaction to shifts to the right. If Kc decreases with a decrease in temperature, the reaction to shifts to the left. If the products dominate in a reaction, the value for K is greater than 1.
What can change KC?
8.2. 3 : The only thing which can change the value of Kc for a given reaction is a change in temperature. The position of equilibrium, however, can change without a change in the value of Kc.
What factors affect KC value?
The only thing which can change the value of Kc for a given reaction is a change in temperature. The position of equilibrium, however, can change without a change in the value of Kc. The effect of a change of temperature on a reaction will depend on whether the forward reaction is exothermic or endothermic.
Le Chatelier’s Principle
Images related to the topicLe Chatelier’s Principle
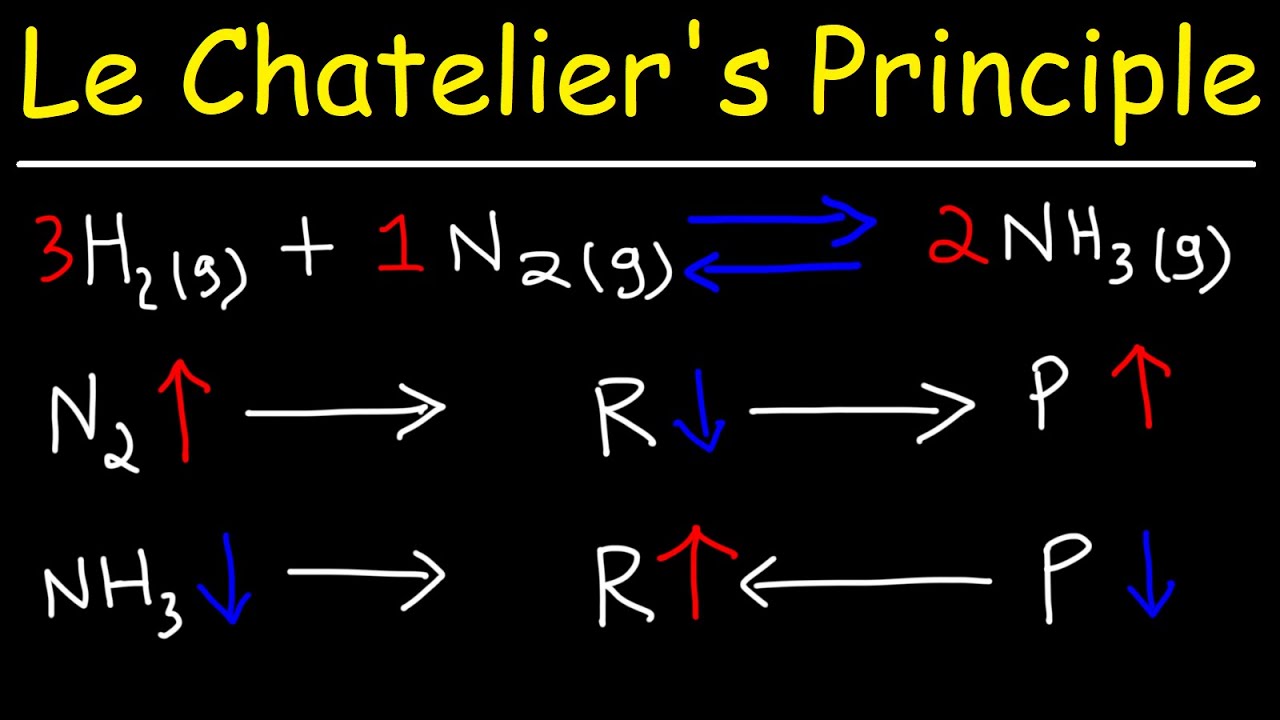
Will the equilibrium constant for the reaction increase decrease or stay the same?
Will the equilibrium constant for the reaction increase, decrease, or stay the same? The equilibrium constant for the reaction will stay the same.
Why does temperature increase rate constant?
Going back to the rate law equation, it follows that a higher rate constant results in a higher reaction rate. This makes sense because as temperature increases, molecules move faster and collide more frequently, resulting in an increased fraction of molecules with higher energy than the activation energy.
How is Ka affected by temperature?
Ka measures the position of equilibrium for the dissociation of an acid. For most acids, the dissociation is an endothermic process. According to Le Châtelier’s Principle, if you add heat to an endothermic process, the position of equilibrium moves to the right. Ka increases!
Is equilibrium constant dependent on temperature?
K is the symbol given to the equilibrium constant for a chemical reaction. The value of the equilibrium constant, K, for a given reaction is dependent on temperature.
Why the value of equilibrium constant depends on temperature?
This is because equilibrium is defined as a condition resulting from the rates of forward and reverse reactions being equal. If the temperature changes, the corresponding change in those reaction rates will alter the equilibrium constant.
How does temperature affect equilibrium endothermic?
An increase in temperature favours the endothermic reaction. In the above equilibrium, the enthalpy change shows that the forward reaction is endothermic. Increasing the temperature will shift the equilibrium to the right hand side.
Does rate constant change with temperature?
The rate constant goes on increasing as the temperature goes up, but the rate of increase falls off quite rapidly at higher temperatures.
Which way does equilibrium shift when temperature is increased?
If the temperature is increasing, a product is being added to the equilibrium, so the equilibrium shifts to minimize the addition of extra product: it shifts back toward reactants.
Matric/Grade 12 Chemistry Past paper 2020 Question 6 Equilibrium
Images related to the topicMatric/Grade 12 Chemistry Past paper 2020 Question 6 Equilibrium

What would happen to a system at equilibrium if the temperature were changed?
When we change the temperature of a system at equilibrium, the equilibrium constant for the reaction changes. Lowering the temperature in the HI system increases the equilibrium constant: At the new equilibrium the concentration of HI has increased and the concentrations of H2 and I2 decreased.
What happens to equilibrium when temperature is increased exothermic?
However, for an exothermic reaction, increasing the temperature decreases the equilibrium constant K.
Related searches to Does the value of KC increase/decrease or remain the same when the temperature is decreased?
- how to tell if a reaction is endothermic or exothermic by looking at the equation
- what happens to equilibrium when temperature is increased
- what happens to equilibrium constant when temperature is increased
- increasing the temperature of an exothermic reaction results in
- how to know if a reaction is endothermic or exothermic without enthalpy
- does the value of kc increase decrease or remain the same when the temperature is decreased
- why is kc only affected by temperature
- how does temperature affect endothermic and exothermic reactions
- increasing the temperature of an endothermic reaction results in
Information related to the topic Does the value of KC increase/decrease or remain the same when the temperature is decreased?
Here are the search results of the thread Does the value of KC increase/decrease or remain the same when the temperature is decreased? from Bing. You can read more if you want.
You have just come across an article on the topic Does the value of KC increase/decrease or remain the same when the temperature is decreased?. If you found this article useful, please share it. Thank you very much.