Are you looking for an answer to the topic “How can you distinguish benzaldehyde from acetaldehyde?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Solution : Acetone `(CH_(3)COCH_(3))` being a methyl ketone gives a positive iodoform test (forms yellow precipitate) but benzaldehyde does not respond to this test. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.Benzaldehyde and formaldehyde can be distinguished by Fehling’s test. Fehling’s solution is an alkaline solution of copper sulphate containing sodium potassium tartarate. When fehling’s solution will be added to formaldehyde , methanoic acid and cuprous oxide (red precipitate ) will be formed.(b) Iodoform test
When acetophenone (a methyl ketone) is oxidised by sodium hypoiodite, it produces a yellow iodoform precipitate (NaOI). Benzaldehyde, on the other hand, does not respond to this test. (vii) Ethanal and propanal can be distinguished by iodoform test.
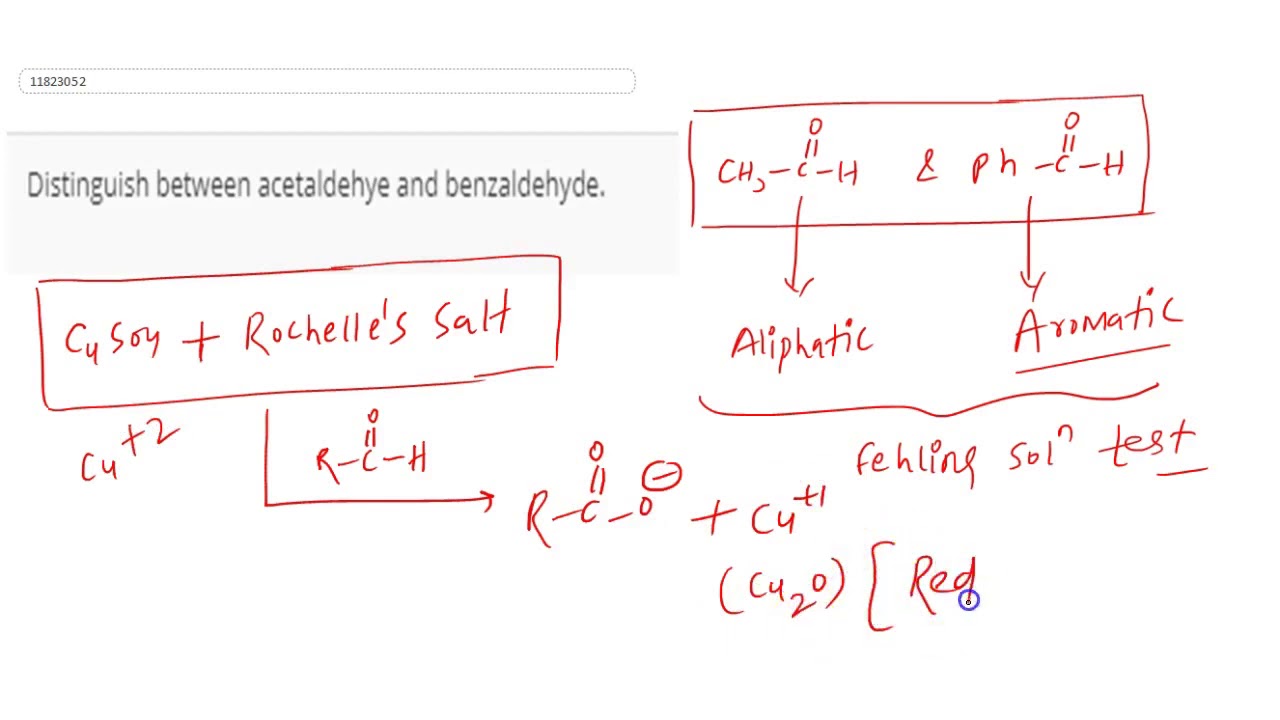
- (i) Distinction between acetaldehyde and benzaldehyde : – Acetaldehyde and benzaldehyde can be distinguish by Fehling solution.
- Acetaldehyde gives red coloured precipitate with Fehling solution while benzaldehyde does not.

Table of Contents
How will you distinguish benzaldehyde from acetaldehyde and acetone?
Solution : Acetone `(CH_(3)COCH_(3))` being a methyl ketone gives a positive iodoform test (forms yellow precipitate) but benzaldehyde does not respond to this test. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
How can you distinguish between formaldehyde and benzaldehyde?
Benzaldehyde and formaldehyde can be distinguished by Fehling’s test. Fehling’s solution is an alkaline solution of copper sulphate containing sodium potassium tartarate. When fehling’s solution will be added to formaldehyde , methanoic acid and cuprous oxide (red precipitate ) will be formed.
Give one chemical test to distinguish between acetaldehyde and benzaldehyde.
Images related to the topicGive one chemical test to distinguish between acetaldehyde and benzaldehyde.

How do you distinguish between benzaldehyde and Ethanal?
(b) Iodoform test
When acetophenone (a methyl ketone) is oxidised by sodium hypoiodite, it produces a yellow iodoform precipitate (NaOI). Benzaldehyde, on the other hand, does not respond to this test. (vii) Ethanal and propanal can be distinguished by iodoform test.
How will you distinguish between acetaldehyde or propanal?
Ethanal and propanal can be distinguished by iodoform test. Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom responds to the iodoform test. Ethanal having one methyl group linked to the carbonyl carbon atom responds to this test.
How can you distinguish between benzaldehyde and acetone?
Solution. Benzaldehyde gives Tollen’s reagent test. i.e., a silver mirror with Tollen’s reagent but acetone does not.
What happens in Fehling’s test?
Principle of Fehling’s Test
On heating, the sample with the Fehling’s solution, bistartarocuprate (II) complex oxidizes the aldoses to corresponding aldonic acids. In the process, the copper (II) ions of the complex are reduced to insoluble yellow or red-colored precipitate or cuprous (I) oxide (Cu2O) ions.
Can Fehling test be used to distinguish between acetaldehyde and benzaldehyde?
Thus Benzaldehyde & Acetaldehyde can be differentiated using Iodoform and Fehling test.
See some more details on the topic How can you distinguish benzaldehyde from acetaldehyde? here:
Difference Between Benzaldehyde and Acetaldehyde
Benzaldehyde: Benzaldehyde is a colorless liquid with an almond-like odor. Acetaldehyde: Acetaldehyde is a colorless liquid with a pungent odor.
Acetaldehyde and benzaldehyde can be differentiated by
Acetaldehyde and benzaldehyde can be differentiated by: A. Fehling’s test. B. Iodoform test. C. Tollens reagent. D. Both ‘A’ and ‘B’.
Give one chemical test to distinguish between the following …
Acetaldehyde and benzaldehyde: Acetaldehyde gives a red precipitate with Fehling’s solution but benzaldehyde does not.
Can Fehling’s test be used to distinguish acetaldehyde and benzaldehyde?
Fehling’s solution can be used to distinguish benzaldehyde from acetaldehyde.
How can you distinguish between formaldehyde and acetaldehyde?
Solution. Acetaldehyde, when heated with I2 and NaOH, gives a yellow precipitate of iodoform (Iodoform test) whereas formaldehyde does not give a yellow precipitate of iodoform.
How can you distinguish between ethanol and benzaldehyde?
Answer. Answer: Add Tollen’s reagent and warm. Benzaldehyde will give a positive test of a silver mirror effect whereas Ethanol gives no colour change.
How will you distinguish between benzaldehyde and benzophenone?
Benzaldehyde is an aromatic aldehyde having the chemical formula C6H5CHO while Benzophenone is an aromatic ketone having the chemical formula C13H10O. In summary, the key difference between benzaldehyde and benzophenone is that benzaldehyde is an aldehyde, whereas benzophenone is a ketone.
How can we distinguish between benzaldehyde and acetophenone?
Tollen’s test – aldehydes give a positive Tollen’s test. Benzaldehyde is an aldehyde. Therefore, it reduces Tollen’s reagent to give a reddish-brown precipitate. Acetophenone is a ketone and therefore gives a negative test.
Distinguish between acetaldehye and benzaldehyde.
Images related to the topicDistinguish between acetaldehye and benzaldehyde.
How will you distinguish between benzaldehyde and benzoic acid?
Solution. Benzaldehyde reacts with ammoniacal solution of silver nitrate to form a silver mirror. However, no such reaction is given by benzoic acid.
What is iodoform test?
Iodoform test is used to check the presence of carbonyl compounds with the structure R-CO-CH3 or alcohols with the structure R-CH(OH)-CH3 in a given unknown substance. The reaction of iodine, a base and a methyl ketone gives a yellow precipitate along with an “antiseptic” smell.
What is the difference between tollens test and Fehling’s test?
Tollen’s Test: Aldehydes give positive Tollen’s test (silver mirror) while ketones do not give any reaction. 2. Fehling’s test: Aliphatic aldehydes on treatment with Fehling’s solution give a reddish brown precipitate (positive result) while aromatic aldehydes and ketones do not. 3.
Which test is very useful method to distinguish between benzaldehyde and acetone?
i) Benzaldehyde gives the tollen test but acetone does not give this test. (ii) Carbylamine test will be given by 1° Amine i.e. methylamine, not by dimethyl amine.
Does benzaldehyde give tollens test?
Benzaldehyde gives a positive test with Tollen’s reagent but not with Fehling’s and Benedict solutionws.
Does benzaldehyde give iodoform test?
Solution : Add a solution of `I_(2)//NaOH` to sample. The sample which gives a yellow precipitate of iodoform `(CHI_(3))` is acetaldehyde. Benzaldehyde does not give this test.
Why does benzaldehyde not respond to Fehling’s test?
Aldehydes such as benzaldehyde, lack alpha hydrogens and cannot form an enolate and thus do not give a positive test with Fehling’s solution which is comparatively a weaker oxidizing agent than Tollen’s reagent, under usual conditions. Therefore, it tests negative. ) as the aldehyde is oxidized to a carboxylic acid.
What is Fehling A and Fehling B?
Fehling A is a blue-colored aqueous solution of copper (II) sulfate (CuSO4). Fehling B is a colorless aqueous solution of potassium sodium tartrate (KNaC4H4O6·4H2O, also known as Rochelle salt) in an alkaline base like sodium hydroxide (NaOH).
What gives positive Fehling’s test?
Only glucose has aldehyde group. So glucose gives positive test for Fehling’s solution.
Does acetaldehyde give Tollens test?
This is what gives the Tollens test named as the silver mirror test. – So, when acetaldehyde reacts with the above reagent, elemental silver precipitates out and acetaldehyde gets oxidized to acetic acid. So, the correct answer is “Option C”.
identification of acetaldehyde from benzaldehyde
Images related to the topicidentification of acetaldehyde from benzaldehyde

Which of the following reagent is used to distinguish between ethanal and benzaldehyde?
Both benzaldehyde and acetaldehyde reduces Tollen’s reagent.
Which of the following tests can distinguish between acetaldehyde and Phenylacetaldehyde?
<br> Iodoform Test.
Related searches to How can you distinguish benzaldehyde from acetaldehyde?
- benzaldehyde and acetaldehyde reaction
- how can you distinguish benzaldehyde from acetaldehyde free
- distinguish between acetophenone and benzophenone
- benzaldehyde iodoform test
- benzaldehyde acetaldehyde aldol condensation
- does acetaldehyde give iodoform test
- benzaldehyde
- how can you distinguish benzaldehyde from acetaldehyde in alcohol
- acetaldehyde iodoform test
- distinguish between propanal and propanone
- how can you distinguish benzaldehyde from acetaldehyde in alcohol fermentation
- how can you distinguish benzaldehyde from acetaldehyde solution
Information related to the topic How can you distinguish benzaldehyde from acetaldehyde?
Here are the search results of the thread How can you distinguish benzaldehyde from acetaldehyde? from Bing. You can read more if you want.
You have just come across an article on the topic How can you distinguish benzaldehyde from acetaldehyde?. If you found this article useful, please share it. Thank you very much.