Are you looking for an answer to the topic “How catalyst increases the rate of reaction explain with the help of potential energy diagram?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Solution. A catalyst provides alternative pathway associated with lower activation energy. The potential energy diagram compares the potential energy barriers for the catalysed and uncatalysed reactions. The barrier for uncatalysed reaction (Ea) is larger than that for the same reaction in the presence of a catalyst Ea …A catalyst provides an alternative reaction pathway which involves less energy and so the catalyst lowers the activation energy. The use of a catalyst does not affect the reactants or products, so stays the same.A catalyst is a substance that can be added to a reaction to increase the reaction rate without getting consumed in the process. Catalysts typically speed up a reaction by reducing the activation energy or changing the reaction mechanism. Enzymes are proteins that act as catalysts in biochemical reactions.
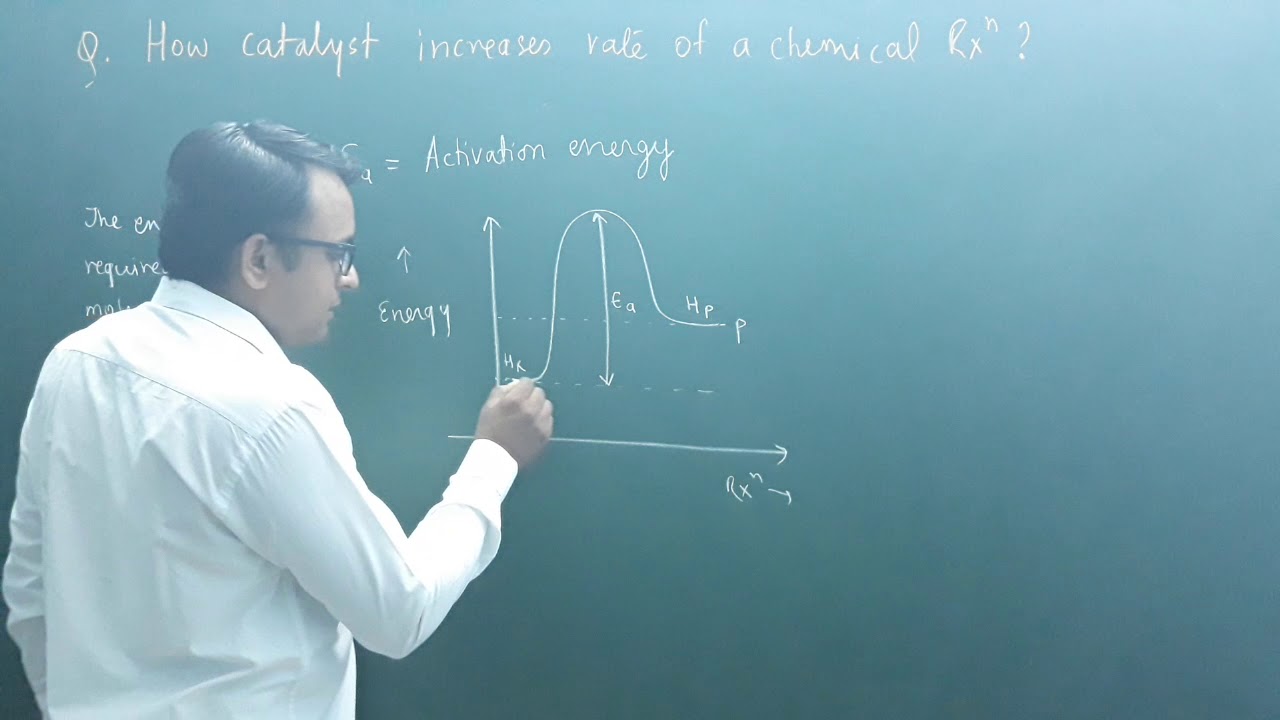
Table of Contents
How does a catalyst affect a potential energy diagram?
A catalyst provides an alternative reaction pathway which involves less energy and so the catalyst lowers the activation energy. The use of a catalyst does not affect the reactants or products, so stays the same.
How does a catalyst increases the rate of reaction?
A catalyst is a substance that can be added to a reaction to increase the reaction rate without getting consumed in the process. Catalysts typically speed up a reaction by reducing the activation energy or changing the reaction mechanism. Enzymes are proteins that act as catalysts in biochemical reactions.
How catalyst increases rate of a chemical reaction?( class 12| chemical kinetics)
Images related to the topicHow catalyst increases rate of a chemical reaction?( class 12| chemical kinetics)

Does a catalyst increase the potential energy of the reactants?
Catalysts have no effect on the change in potential energy for a reaction.
How does a catalyst increase the rate of reaction Mcq?
A catalyst increases the rate of a reaction by lowering the activation energy so that more reactant molecules collide with enough energy to surmount the smaller energy barrier.
How do catalysts increase the rate of a chemical reaction Brainly?
⭐ CATALYST : A catalyst is an agent or compound that is added to a process to make a chemical reaction happen more quickly. ✨A catalyst increases the rate of the reaction because: They provide an alternative energy pathway that has a lower activation energy.
How does a catalyst increase the rate of a reaction quizlet?
Catalysts increase the rate of reaction without being used up. They do this by lowering the activation energy needed. With a catalyst, more collisions result in a reaction, so the rate of reaction increases.
What is potential energy diagram?
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. Potential energy diagrams for endothermic and exothermic reactions are described. Diagrams of activation energy and reaction progress are given.
See some more details on the topic How catalyst increases the rate of reaction explain with the help of potential energy diagram? here:
Answer the following in brief. How a catalyst increases …
ii. The potential energy diagram compares the potential energy barriers for the catalysed and uncatalysed reactions. The barrier for uncatalysed reaction (Ea) …
How catalyst increases the rate of reaction? Explain with the …
(i) A catalyst is a substance, when added to the reactants, increases the rate of the reaction without being consumed.
How Do Catalysts Increase The Rate Of A Chemical Reaction
How catalyst increases the rate of reaction explain with the help of potential energy diagram for catalysed and and catalysed reactions?
7.4 Mechanism of reaction and catalysis – Siyavula
A catalyst speeds up a chemical reaction, without being consumed by the reaction. It increases the reaction rate by lowering the activation energy for a …
What is an energy diagram?
Energy diagrams are diagrams that show the amount of relative potential energy in each step of a reaction. Energy diagrams typically include: the reactants, transition states, and products (although depending on the reaction can include more information such as intermediates, activation energy, etc.)
Potential Energy Diagrams – Chemistry – Catalyst, Endothermic Exothermic Reactions
Images related to the topicPotential Energy Diagrams – Chemistry – Catalyst, Endothermic Exothermic Reactions

How does a catalyst increase the rate of a reaction GCSE?
A catalyst provides an alternative reaction pathway that has a lower activation energy than the uncatalysed reaction. This does not change the frequency of collisions. However, it does increase the frequency of successful collisions because a greater proportion of collisions now exceeds this lower activation energy.
What is a reaction pathway diagram?
For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a single energetic pathway, along the reaction coordinate, as the reactants are transformed into products.
Does adding more catalyst increase reaction rate?
A catalyst increases the rate of reaction in a slightly unconventional way from other means of increasing reaction rate. The role of a catalyst is to lower the activation energy so that a greater proportion of the particles have enough energy to react.
Which statement is true about the potential energy diagram for an endothermic reaction?
Answer: Products have less potential energy than reactants. In an endothermic reaction, the reaction mixture will absorb heat from the surroundings. Therefore, the products will have higher energy than the reactants and hence, ΔH will be positive.
Which describes the reaction shown in the potential energy profile?
Q. Which describes the reaction shown in the potential energy profile? The reaction is endothermic and the products have greater enthalpy than the reactants.
What is a catalyst and how does it affect the rate of reaction?
The rate of a reaction can be increased by adding a suitable catalyst. A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically unchanged at the end). It provides an alternative reaction pathway of lower activation energy.
Which of the following is the reason s why the catalysts increase the rate of a reaction hint catalysts only lower the activation energy ): More than one answer?
Correct answer:
A catalyst has no effect on the relative stability of the reactants or products, nor does it effect the temperature of a reaction. Instead, catalysts lower the energy of transition states, increasing their stability, to lower the overall activation energy of the reaction.
Catalyst Affects Reaction Rate – Energy Diagram with a Catalyst
Images related to the topicCatalyst Affects Reaction Rate – Energy Diagram with a Catalyst

What is the role of a catalyst in a chemical reaction Mcq?
The role of a catalyst in a reaction is to lower the activation energy of the reactants.
How does a catalyst work Mcq?
Catalyst is a substance that increases the rate of reaction and the process is known as catalysis. Berzelius coined the term ‘catalyst’ in 1835. Catalysts affect the forward and backward reactions equally so they do not alter the value of the equilibrium constant.
Related searches to How catalyst increases the rate of reaction explain with the help of potential energy diagram?
- define order of reaction with suitable examples.
- explain potential energy diagram
- how will you represent first order reaction graphically
- how catalyst increases the rate of reaction explain with the help of potential energy diagram
- for a zero order reaction molecularity can never be equal to zero. explain
- for a zero order reaction molecularity can never be equal to zero explain
- derive integrated rate law for gas phase reaction
- define order of reaction with suitable examples
- define first order reaction with unit
- explain graphically the effect of temperature on the rate of reaction
- derive the integrated rate law for first order reaction
Information related to the topic How catalyst increases the rate of reaction explain with the help of potential energy diagram?
Here are the search results of the thread How catalyst increases the rate of reaction explain with the help of potential energy diagram? from Bing. You can read more if you want.
You have just come across an article on the topic How catalyst increases the rate of reaction explain with the help of potential energy diagram?. If you found this article useful, please share it. Thank you very much.