Are you looking for an answer to the topic “How do cathode rays differ from anode rays?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Cathode rays contain material particles (electrons) which are negatively charged. Anode rays contain material particles which are positively charged. These rays are deflected in both magnetic and electric fields. These rays are deflected in both magnetic and electric fields.But the particles in cathode rays are negatively charged and they are electrons while those in anode rays are positively charged and they are protons. When the cathode rays are passed through the electric field, they bend towards anode and anode rays bend towards the cathode.Cathode rays are the beam of electrons travelling from the negatively-charged cathode to the positively charged anode at the other end of the vacuum tube. These cathode rays travel in a straight-line path at high speed when a voltage difference is applied to the electrodes.
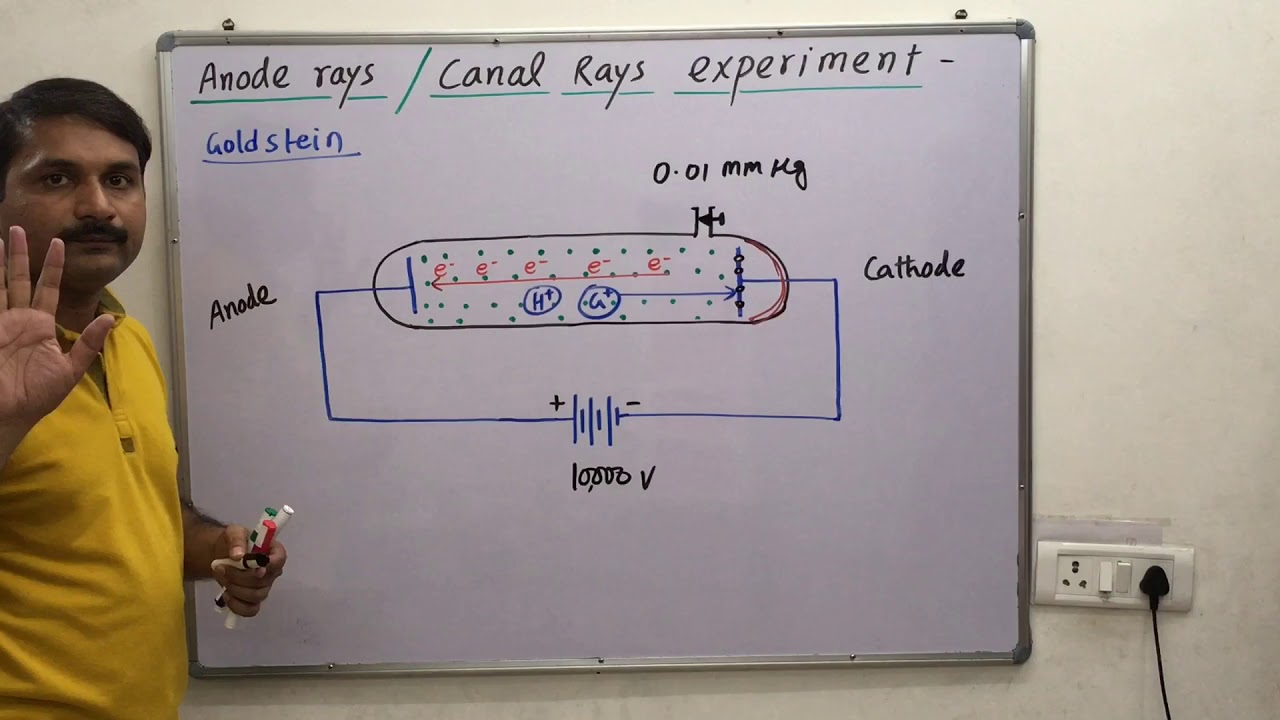
Table of Contents
How do cathode rays differ from anode rays class 9?
But the particles in cathode rays are negatively charged and they are electrons while those in anode rays are positively charged and they are protons. When the cathode rays are passed through the electric field, they bend towards anode and anode rays bend towards the cathode.
What are the properties of cathode and anode rays?
Cathode rays are the beam of electrons travelling from the negatively-charged cathode to the positively charged anode at the other end of the vacuum tube. These cathode rays travel in a straight-line path at high speed when a voltage difference is applied to the electrodes.
2.3 Anode Ray’s / Canal Ray’s and it’s characteristics, / discovery of proton /Atomic structure
Images related to the topic2.3 Anode Ray’s / Canal Ray’s and it’s characteristics, / discovery of proton /Atomic structure

What is the difference between cathode rays and electrons?
Cathode rays are a beam of electrons emerging from the cathode (the negative terminal) within an electron tube. Electrons are actual particles with mass and charge.
Why are anode rays different?
Different gases give different types of anode rays in the discharge tube experiment because the mass and charge of the anode rays particles depend on the nature of the gas taken in the discharge tube.
What is cathode and anode?
The Anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction.
What are the differences in the discharge tube used to study cathode rays and anode rays?
A cathode ray discharge tube is essentially a glass tube with closed ends. Two metal plates which serve as cathode and anode are sealed at the two ends of the glass tube. The discharge tube used to study anode rays has the perforated plate serving as cathode and is sealed in the middle of the glass tube.
What are the 4 properties of cathode rays?
Property 1: Cathode rays travel in a straight line and they can cast sharp shadows. Property 2: They are negatively charged. Property 3: Electric and magnetic fields deflect cathode rays. Property 4: They are produced at the cathode and travel to the anode in a vacuum tube.
See some more details on the topic How do cathode rays differ from anode rays? here:
How do cathode rays differ from anode rays ? – BYJU’S
Cathode rays: 1. They have negatively charged ions called electrons. 2. Cathode rays deflect towards the positive plate of an electric field.
How does cathode ray differ from as anode rays? – Chemistry
Cathode rays. Anode rays ; These rays travel from the cathode to the anode. These rays travel from the anode to the cathode. ; They are made of negatively charged …
How does cathode rays differ from the anode rays – Brainly.in
Cathode rays: 1. They have negatively charged ions called electrons. 2. Cathode rays deflect towards the positive plate of an electric field …
What are the two properties of anode rays?
- Anode rays deflect towards negatively charged plate.
- Anode rays travel along a straight path in absence of electric and magnetic field.
- Anode rays are positively charged radiations due to positive charged sub-atomic particles.
What are the major characteristics of cathode rays?
- Travel at straight line in vaccum.
- it has energy and momentum.
- negatively charged.
- it can ionize air or gaseous.
- cathode rays possess kinetic energy.
What is the difference between cathode rays and alpha particles?
…
| Cathode rays | α− rays |
|---|---|
| Cathode rays are negatively charged. | α− rays are positively charged. |
| Cathode rays are produced from a metal plate which can act as a cathode when connected to a high voltage electric circuit. | α− rays emitted from a radioactive element. |
Cathode Ray Tube | Definition | Charatersitics | Diagram
Images related to the topicCathode Ray Tube | Definition | Charatersitics | Diagram

Is cathode positive or negative?
The cathode is the electrode where electricity is given out or flows out. The anode is usually the positive side. A cathode is a negative side. It acts as an electron donor.
Is a cathode ray positive or negative?
The cathode ray is composed of negatively-charged particles.
Why are anode rays called cathode rays?
Anode ray tube
These rays are beams of particles moving in a direction opposite to the “cathode rays”, which are streams of electrons which move toward the anode. Goldstein called these positive rays Kanalstrahlen, “channel rays”, or “canal rays”, because they were produced by the holes or channels in the cathode.
What are cathode rays?
cathode ray, stream of electrons leaving the negative electrode (cathode) in a discharge tube containing a gas at low pressure, or electrons emitted by a heated filament in certain electron tubes.
What is cathode ray and anode Ray?
Cathode rays contain material particles (electrons) which are negatively charged. Anode rays contain material particles which are positively charged. These rays are deflected in both magnetic and electric fields. These rays are deflected in both magnetic and electric fields.
What is the difference between anode and anion?
anode is an electrode that is positively charged. anion is negatively charged ion.
What is the nature of the rays Travelling from cathode to the anode in the discharge tube?
Answer: Cathode rays are produced by negative electrode towards anode. They travel in straight lines and give sharp shadows. They can be deflected by only electric and magnetic fields.
What is a cathode ray made of?
What are cathode rays made of? Thomson showed that cathode rays were composed of a negative charged particle, previously unknown, which was later named electron. To render an image on a screen, Cathode ray tubes (CRTs) use a focused beam of electrons deflected by electrical or magnetic fields.
How do cathode rays differ from anode rays? | 9 | ATOMIC STRUCTURE AND CHEMICAL BONDING | CHEMIS…
Images related to the topicHow do cathode rays differ from anode rays? | 9 | ATOMIC STRUCTURE AND CHEMICAL BONDING | CHEMIS…
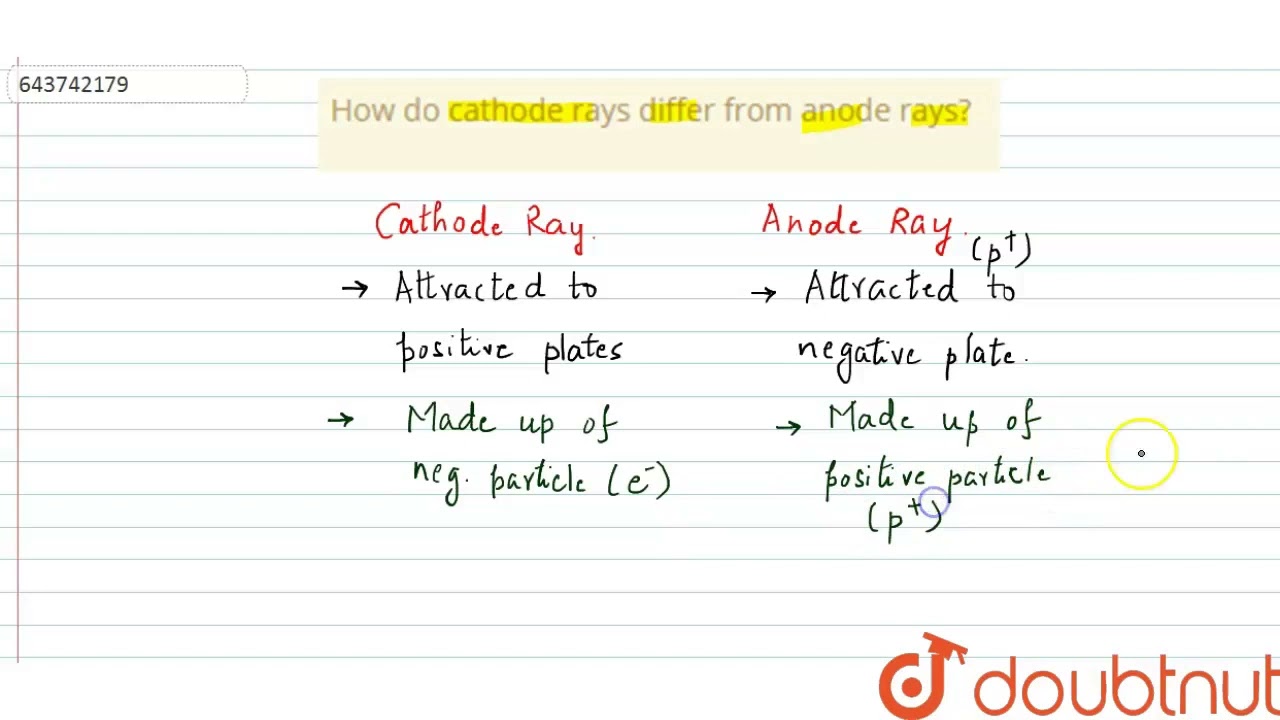
How are anode rays produced?
Anode rays are not emitted from the anode but are produced in the space between the anode and the cathode. The beam of rays which travel in a direction away from anode towards cathode when gas is taken in a discharge tube is subjected to the action of high voltage under low pressure is known as canal rays.
What are anode rays class 9?
Canal rays (or anode rays) are streams of positively charged particles which move towards the negative electrode (cathode) in a discharge tube when high voltage electricity is passed through a gas at very low pressure taken in the discharge tube.
Related searches to How do cathode rays differ from anode rays?
- what are cathode rays and how do they differ from positive rays
- give one property in which cathode rays and anode rays differ
- cathode rays and anode rays class 9
- what are anode rays class 9
- what is the difference between cathode rays and canal rays
- what are anode rays
- how do cathode rays differ from anode rays class 9
- similarities between cathode and anode rays
- properties of anode rays
- what is wrong about anode rays
- what is anode rays
- how do cathode rays differ from anode rays
- are cathode rays dangerous
- properties of cathode rays and anode rays
- what is cathode rays
- why are anode rays called so
- difference between cathode rays and anode rays class 11
Information related to the topic How do cathode rays differ from anode rays?
Here are the search results of the thread How do cathode rays differ from anode rays? from Bing. You can read more if you want.
You have just come across an article on the topic How do cathode rays differ from anode rays?. If you found this article useful, please share it. Thank you very much.