Are you looking for an answer to the topic “How do sodium and chlorine atoms form the ions in sodium chloride and how the ions are arranged in the solid sodium chloride?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
A sodium atom loses an electron to a chlorine atom. The sodium atom becomes a positive sodium ion. The chlorine atom becomes a negative chloride ion. Both sodium ions and chloride ions have full electron shells.Sodium and chloride form an ionic bond. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. As this happens, the electron is transferred from the sodium atom to the chloride atom and so both atoms become ionic and have a full outer shell.The ions in a compound , such as sodium chloride, are arranged in a giant ionic structure (also known as a giant ionic lattice). This regular arrangement results in the formation of a crystal .
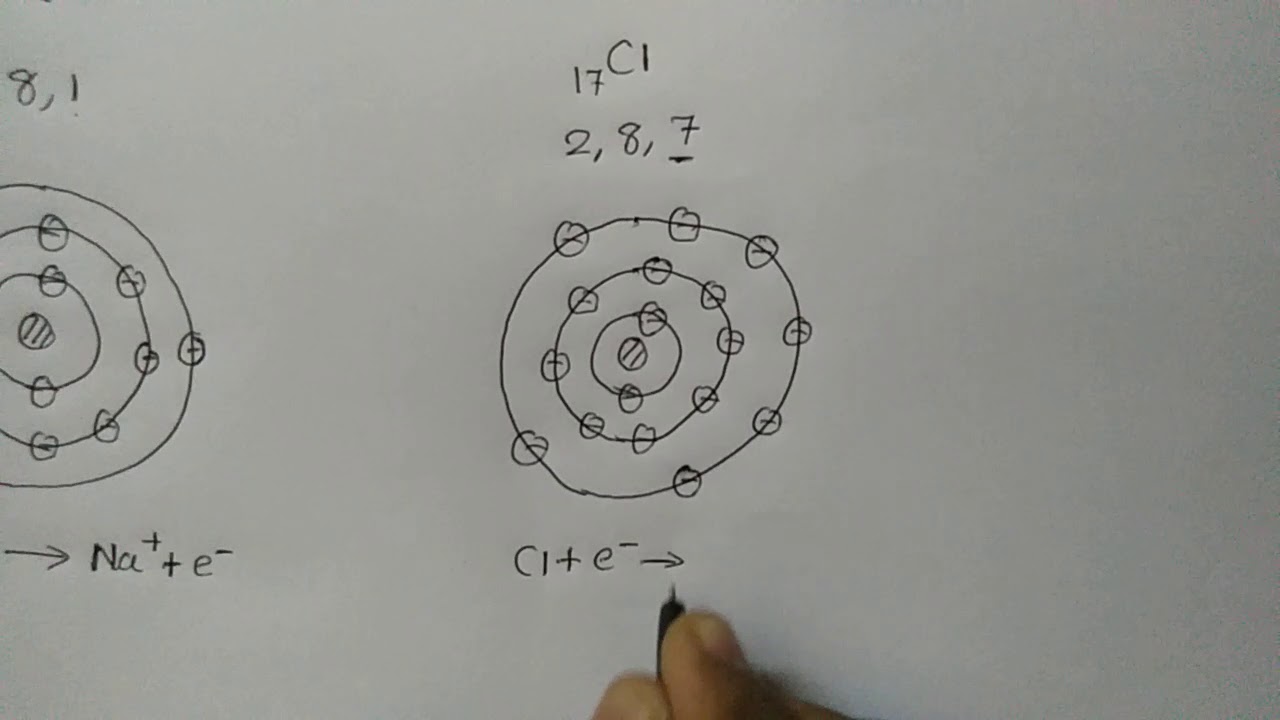
Table of Contents
How do sodium and chlorine atoms form the ions in sodium chloride?
Sodium and chloride form an ionic bond. Therefore the sodium atom loses one electron from its outer shell and the chlorine atom gains one electron. As this happens, the electron is transferred from the sodium atom to the chloride atom and so both atoms become ionic and have a full outer shell.
How are ions arranged in sodium chloride?
The ions in a compound , such as sodium chloride, are arranged in a giant ionic structure (also known as a giant ionic lattice). This regular arrangement results in the formation of a crystal .
Ionic Bond in Sodium Chloride NaCl Std 9 10
Images related to the topicIonic Bond in Sodium Chloride NaCl Std 9 10

How does an ionic bond form between sodium and chlorine?
An ionic bond results from the electrostatic attraction between these ions. The chlorine atom is much more electronegative than the sodium atom; therefore, when they react, sodium gives up an electron to chlorine to yield sodium (Na+) and chloride (Cl−) ions, which then form an ionic bond.
How does a sodium atom form a sodium ion?
A sodium atom has one electron in the outer shell. A chlorine atom seven electrons in the outer shell. A sodium atom loses an electron to a chlorine atom. The sodium atom becomes a positive sodium ion.
How does chlorine atom become a chloride ion?
Chlorine is in Group 7. It has seven electrons in its outer shell. It gains an electron from another atom in reactions, forming a chloride ion, Cl –.
How are the particles arranged and how do the particles move in sodium chloride at 900 C?
The particles are arranged in the following manner:
Sodium Chloride (NaCl) is made of Na+ ions and Cl- ions. At 900°C, sodium chloride exists in liquid form (molten form). The ions are free to move and show random movement. This is the reason why molten NaCl conducts electricity.
What type of structure and bonding does solid sodium chloride have?
Answer. Sodium chloride has a giant ionic lattice. This is formed due to the ionic bonding, which involves the transfer of electrons from one atom to another to ensure each atom has a full outer shell of electrons. Sodium will lose an electron to become Na+ whilst chloride will gain an electron to form Cl-.
See some more details on the topic How do sodium and chlorine atoms form the ions in sodium chloride and how the ions are arranged in the solid sodium chloride? here:
Chemical Bonds
Familiar compounds include common table salt (Sodium Chloride) and water. Table salt is made from a combination of atoms of sodium (Na) and chlorine (Cl) in a …
Demonstrations – Sodium + Chlorine – Angelo State University
When a sodium atom transfers an electron to a chlorine atom, forming a sodium cation (Na+) and a chloride anion (Cl-), both ions have complete valence …
Energy Levels, Electrons, and Ionic Bonding | Chapter 4
In reality, the chlorine atom would be bonded to another chlorine atom as part of the gas Cl2. The sodium atom would be one of billions of trillions of sodium …
4.6 Ionic bonding – Chemistry, life, the universe and everything
So when sodium metal (Na) reacts with chlorine (Cl2) gas, sodium and chloride ions are produced. In the solid state, these ions are strongly attracted to …
How are ionic bonds structured?
An ionic compound is a giant structure of ions. The ions have a regular, repeating arrangement called an ionic lattice . The lattice is formed because the ions attract each other and form a regular pattern with oppositely charged ions next to each other.
Structure of Sodium Chloride (NaCl)
Images related to the topicStructure of Sodium Chloride (NaCl)
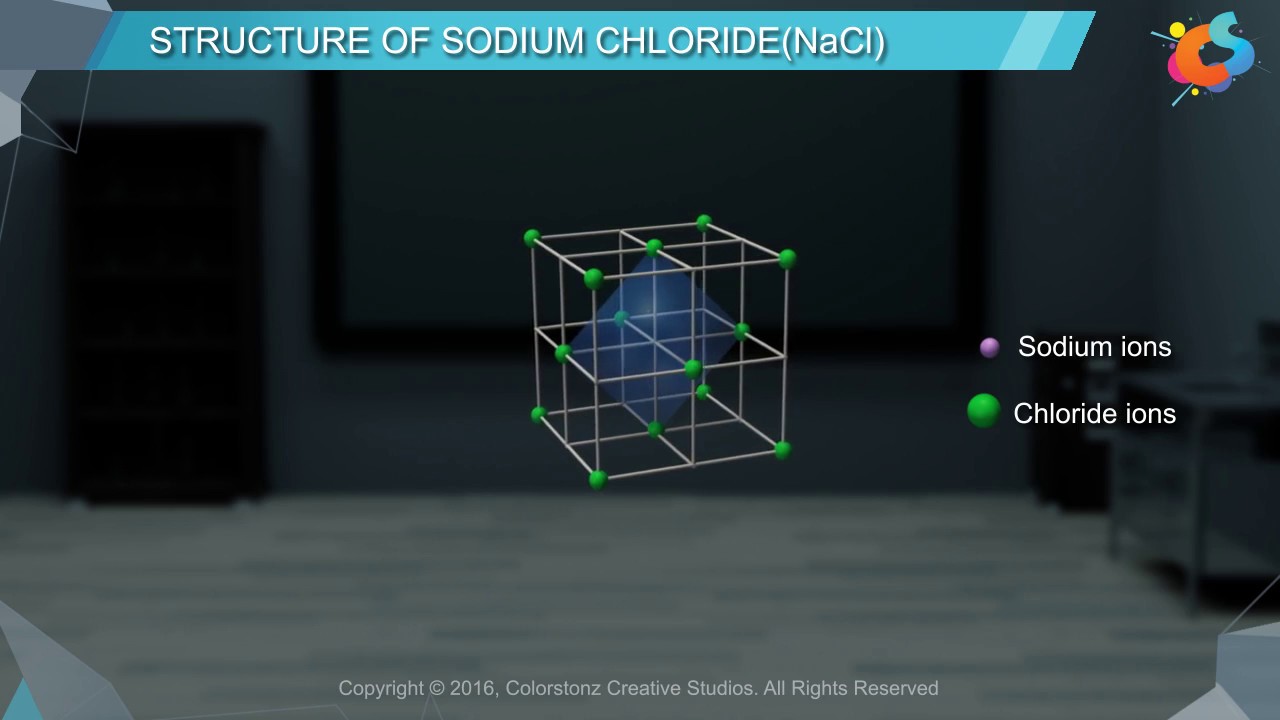
How is sodium chloride formed?
Sodium chloride forms when a sodium atom interacts with chlorine atoms and the sodium donates a negatively charged electron to the chlorine. This process makes sodium positively charged and chlorine negatively charged.
Which kind of bond would occur between sodium and chlorine?
Ionic bonds form when atoms transfer electrons between each other, forming ions that are electrically attracted to each other forming a bond between them. Sodium chloride (NaCl) is a typical ionic compound. The picture below shows both a sodium and a chlorine ion.
How does ionic bonding take place answer?
ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
What is the electron arrangement of a sodium atom?
How is chlorine ion formed?
The chloride ion /ˈklɔːraɪd/ is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents.
How do atoms form ions?
Ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; by combination of ions with other particles; or by rupture of a covalent bond between two atoms in such a way that both of the electrons of the bond are left in association with one of the …
When an atom of chlorine forms an ionic bond with an atom of sodium the atom of chlorine?
When an atom of chlorine forms an ionic bond with an atom of sodium, the atom of chlorine gains a electron to form an anion. Explanation: An ionic bond is formed when an element completely transfers its valence electron to another element.
How Ionic Bonds Form (Basic)
Images related to the topicHow Ionic Bonds Form (Basic)

What is required to separate the sand from the sodium chloride?
Separating Sand and Salt
Probably the easiest method to separate the two substances is to dissolve salt in water, pour the liquid away from the sand, and then evaporate the water to recover the salt.
Which substance is a liquid at 900 C?
1 Sodium chloride is a liquid at 900°C.
Related searches to How do sodium and chlorine atoms form the ions in sodium chloride and how the ions are arranged in the solid sodium chloride?
- sodium chloride is a combination of sodium and
- what type of bond is sodium chloride
- sodium chloride ionic bond diagram
- what happens when sodium reacts with chlorine
- in the example of ionic bond formation between sodium and chlorine
- when sodium and chlorine combine to form sodium chloride, sodium chloride is the:
- when sodium and chlorine combine to form sodium chloride sodium chloride is the
- sodium chloride formula
- sodium chlorine sodium chloride balanced equation
Information related to the topic How do sodium and chlorine atoms form the ions in sodium chloride and how the ions are arranged in the solid sodium chloride?
Here are the search results of the thread How do sodium and chlorine atoms form the ions in sodium chloride and how the ions are arranged in the solid sodium chloride? from Bing. You can read more if you want.
You have just come across an article on the topic How do sodium and chlorine atoms form the ions in sodium chloride and how the ions are arranged in the solid sodium chloride?. If you found this article useful, please share it. Thank you very much.