Are you looking for an answer to the topic “How do you convert 12g of oxygen gas to moles?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Therefore, 12 g of oxygen gas has 0.375 moles.Therefore, 12 g of oxygen gas has 0.375 moles.This means that the atomic mass or atomic weight (12 grams) of carbon is equal to exactly 1 mole of carbon.
- Given mass of O 2 = m = 12g.
- Molar Mass of O 2 = M = 32g.
- Given mass of H 2 O = m = 20g.
- Molar Mass of H 2 O = M = 18g.
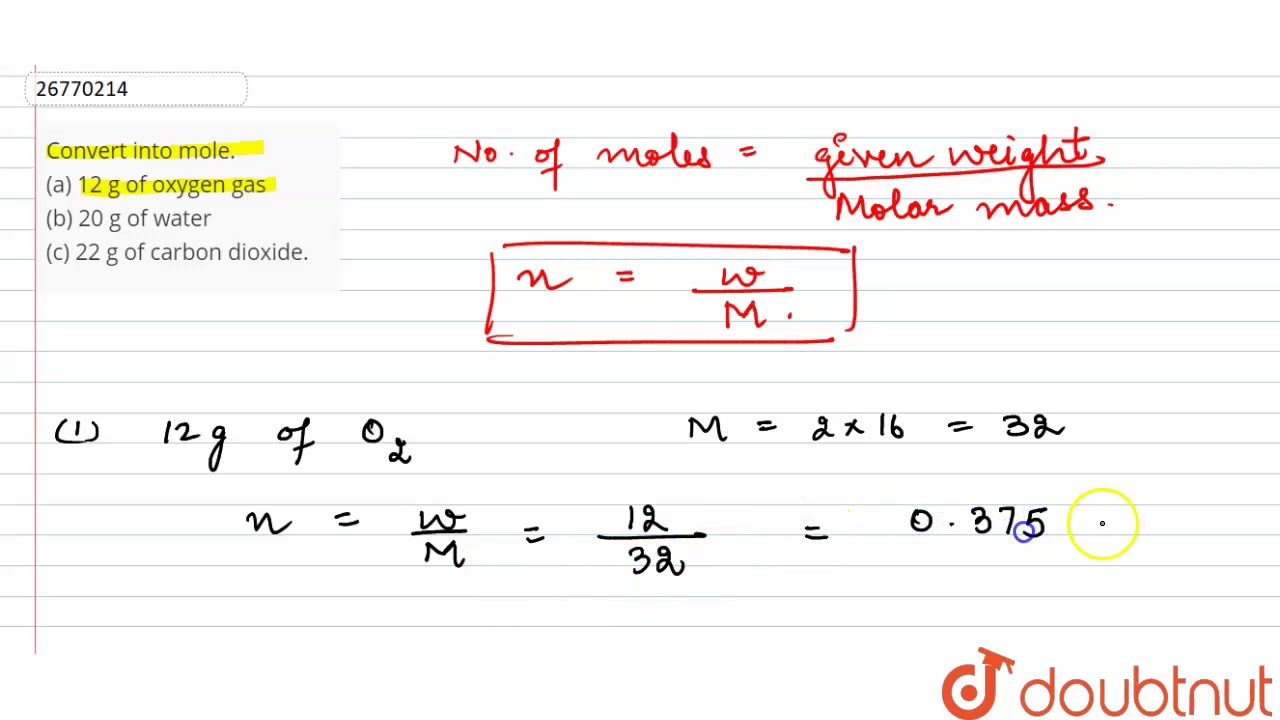
Table of Contents
How do you convert 12g of oxygen to moles?
Therefore, 12 g of oxygen gas has 0.375 moles.
How do you convert 12g of oxygen to gas?
- Given mass of O 2 = m = 12g.
- Molar Mass of O 2 = M = 32g.
- Given mass of H 2 O = m = 20g.
- Molar Mass of H 2 O = M = 18g.
Convert into mole. (a) 12 g of oxygen gas (b) 20 g of water (c) 22 g of carbon dioxide….
Images related to the topicConvert into mole. (a) 12 g of oxygen gas (b) 20 g of water (c) 22 g of carbon dioxide….

How many moles are in 12g?
This means that the atomic mass or atomic weight (12 grams) of carbon is equal to exactly 1 mole of carbon.
What is the molar mass of 12g of oxygen gas?
⇒ 1 Mole of Oxygen has 16 × 2 = 32 grams. Hence, the 12g of Oxygen contains . 375 mol.
How do I calculate moles?
To correctly estimate the number of moles, n , of a substance of a specific mass, m , (in grams), you need to follow the grams to moles formula: n = m / M , where, M is the molar mass of this material.
How do you find the moles of oxygen?
Calculate the number of oxygen atoms in 1 mole of O2. Solution — 1 molecule of O2 = 2 oxygen atoms So, 1 mole of O2 = 2 mole oxygen atoms = 2 × 6.022 × 1023 = 12.044 ×1023 oxygen atoms.
How many moles are in 22g of oxygen gas?
20 times that of avogadro’s constant. Moles of O2= 20/32 = 0.625mol. The 0.625 moles of O2 = 4/7 X 0.625= 0.36moles of CO2.
See some more details on the topic How do you convert 12g of oxygen gas to moles? here:
[Answered] convert into moles 12 G of Oxygen gas – Brainly.in
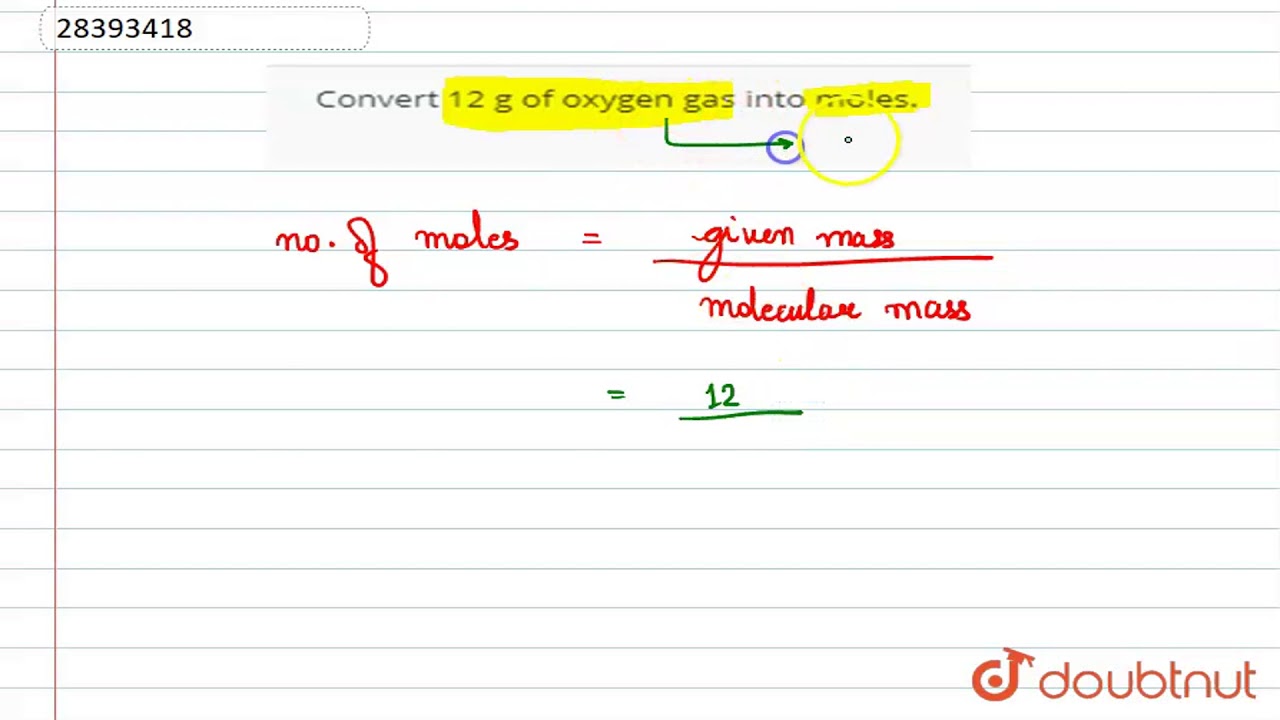
Here we are given the mass of oxygen gas as 12 grams. … Molar mass = 2×16 = 32 grams. We know the formula to calculate the number of moles. … = 0.375 moles.
Convert 12 G of Oxygen Gas into Moles. – Science – Shaalaa …
Hence, 12 g of oxygen contains 0.375 moles of oxygen. Concept: Molecular Mass. Report Error Is there an error in this question or solution?
Convert into mole a 12 g of oxygen gas b 20 g of w – Tutorix
(a) Given mass of oxygen gas = 12 g. Molar mass of oxygen gas (O2) = 32 g. Mole of oxygen gas 12/32 = 0.375 mole. (b) Given mass of water = 20 g.
How many moles are present in 128 g of oxygen molecules?
The number of mole present in 128 g of oxygen is 16.
How do you convert 22g of CO2 to moles?
So, 22 gm of CO2 = 4422 moles of CO2 = 0.5 moles of CO2.
Convert into mole. (a) 12 g of oxygen gas (b) 20 g of water (c) 22 g of carbon dioxide
Images related to the topicConvert into mole. (a) 12 g of oxygen gas (b) 20 g of water (c) 22 g of carbon dioxide

How do I find moles from grams?
Divide the mass of the substance in grams by its molecular weight. This will give you the number of moles of that substance that are in the specified mass. For 12 g of water, (25 g)/(18.015 g/mol) = 0.666 moles.
How many moles are in oxygen?
One mole of oxygen gas, which has the formula O2, has a mass of 32 g and contains 6.02 X 1023 molecules of oxygen but 12.04 X 1023 (2 X 6.02 X 1023) atoms, because each molecule of oxygen contains two oxygen atoms.
How many moles are in 2g of oxygen?
We know that 2g of O2 has 0.0625 moles of O2. That means that the number of O2 molecules present in 2g of O2 is equal to 0.0625*NA.
How many grams are in a mole?
The molar mass of atoms of an element is given by the standard relative atomic mass of the element multiplied by the molar mass constant, 1 × 10−3 kg/mol = 1 g/mol.
How did you convert moles to grams?
The amount of grams in a mole depends on the substance you have. To work it out, find the atomic or molecular mass of your substance and multiply it by the number of moles you have. For one mole, the atomic or molecular mass will be the same as the weight.
How many moles are in 16g of oxygen?
Here, it is given that: 16 g of oxygen contains 1 mole of oxygen atoms. In simple terms we can say that: mass of 1 mole oxygen atom (W) = 16 g / mol.
How many moles are in 10 grams of oxygen?
Hence, there are 0.3125 moles of oxygen are present in 10 g of oxygen gas.
How do you convert 20 grams of water to moles?
Therefore, 20g of water =20/18=1. 11 mole.
Convert 12 g of oxygen gas into moles.
Images related to the topicConvert 12 g of oxygen gas into moles.
What is mass of 0.2 mole of oxygen?
-To calculate the mass of 0.2 moles of oxygen atom we should know the mass of a single atom of oxygen that is 16. -Therefore, the mass of 0.2 moles of oxygen atom is 3.2 grams and the mass of 0.5 moles of a water molecule is 9.0 grams.
How do you find moles of oxygen in CO2?
To calculate a molar mass, we simply sum up the contributions of each element or atom. For carbon dioxide, CO2, one carbon atom contributes 12.01 g/mol, the two oxygens together contribute (2)(16.00) = 32.00 g/mol.
Related searches to How do you convert 12g of oxygen gas to moles?
- convert into mole. (a) 12 g of oxygen gas (b) 20 g of water
- how do you convert 12g of oxygen gas to moles of oxygen
- how do you convert 12g of oxygen gas to moles not at stp
- what is the mass of 0 2 mole of oxygen atom
- convert 12g of oxygen gas into mole class 9
- how do you convert 12g of oxygen gas to moles of co2
- how do you convert 12g of oxygen gas to moles of water
- convert into mole 22 gram of carbon dioxide
- convert into mole a 12 g of oxygen gas b 20 g of water
- how do you convert 12g of oxygen gas to moles calculator
- convert into mole 32 gram of oxygen gas
- convert into mole 12 gram of carbon dioxide
- convert 12 gram of oxygen gas into moles
- convert into mole 20 gram of water
Information related to the topic How do you convert 12g of oxygen gas to moles?
Here are the search results of the thread How do you convert 12g of oxygen gas to moles? from Bing. You can read more if you want.
You have just come across an article on the topic How do you convert 12g of oxygen gas to moles?. If you found this article useful, please share it. Thank you very much.