Are you looking for an answer to the topic “How do you do Stoich?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Keep Reading
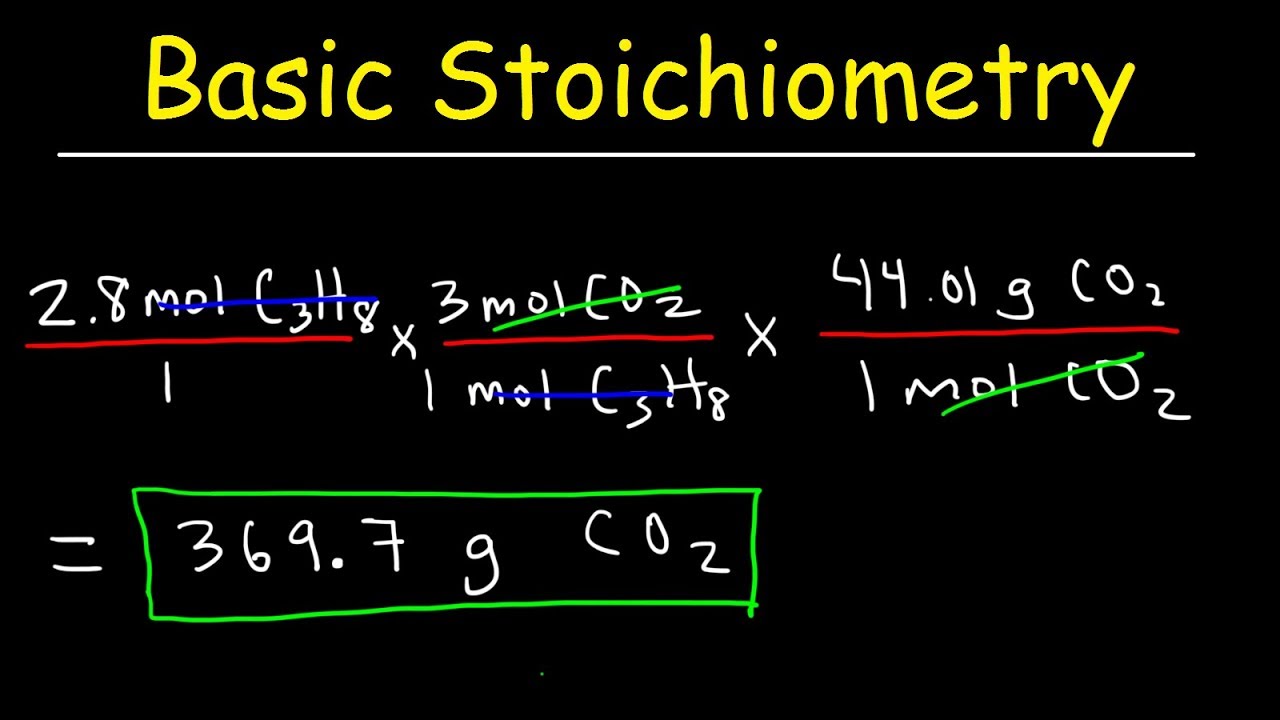
Table of Contents
How do you do Stoich equations?
- Balance the equation.
- Convert units of a given substance to moles.
- Using the mole ratio, calculate the moles of substance yielded by the reaction.
- Convert moles of wanted substance to desired units.
How do you find Stoich ratio?
Thus, to calculate the stoichiometry by mass, the number of molecules required for each reactant is expressed in moles and multiplied by the molar mass of each to give the mass of each reactant per mole of reaction. The mass ratios can be calculated by dividing each by the total in the whole reaction.
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
Images related to the topicStoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems

Why is stoichiometry so hard?
Stoichiometry can be difficult because it builds upon a number of individual skills. To be successful you must master the skills and learn how to plan your problem solving strategy. Master each of these skills before moving on: Calculating Molar Mass.
How do you do a 3 step stoichiometry?
- Step 1: Convert known reactant mass to moles. …
- Step 2: Use the mole ratio to find moles of other reactant. …
- Step 3: Convert moles of other reactant to mass.
How do I calculate moles?
- The formula for the number of moles formula is expressed as.
- Given.
- Number of moles formula is.
- Number of moles = Mass of substance / Mass of one mole.
- Number of moles = 95 / 86.94.
How do you calculate dilution in chemistry?
- C1 is the concentration of the starting solution.
- V1 is the volume of the starting solution.
- C2 is the concentration of the final solution.
- V2 is the volume of the final solution.
How do you calculate Litres of gas?
Molar volume at STP can be used to convert from moles to gas volume and from gas volume to moles. The equality of 1mol=22.4L is the basis for the conversion factor.
See some more details on the topic How do you do Stoich? here:
Stoichiometric Calculations | SparkNotes
Applying Conversion Factors to Stoichiometry · Balance the equation. · Convert units of a given substance to moles. · Using the mole ratio, calculate the moles of …
Stoichiometry (article) | Chemical reactions | Khan Academy
Example: Using mole ratios to calculate mass of a reactant · Step 1: Convert known reactant mass to moles · Step 2: Use the mole ratio to find moles of other …
Stoichiometry and Balancing Reactions – Chemistry LibreTexts
Stoichiometry is a section of chemistry that involves using relationships between reactants and/or products in a chemical reaction to determine …
How to Make Stoichiometry Easy – Sciencing
Multiply the moles of hydrogen by the appropriate stoichiometric ratio to determine the number of moles of any other substance involved in the …
Step by Step Stoichiometry Practice Problems | How to Pass Chemistry
Images related to the topicStep by Step Stoichiometry Practice Problems | How to Pass Chemistry

How does Graham’s law explain why we smell odor?
It states that gas particles are constantly moving in random, rapid motion. So, if you have enough particles (or in this case, cookie smell molecules) in one central location, they will eventually spread out because they are moving randomly and rapidly.
What is a good air/fuel ratio?
In a perfect world, all gasoline engines would run the ideal air-fuel mixture of 14.7 parts air to 1 part fuel. This target mixture, which is referred to as the stoichiometric air-fuel ratio, is a compromise between optimum fuel economy and optimum power output.
What is the point of stoichiometry?
Stoichiometry is the calculation of quantities in chemical equations. Given a chemical reaction, stoichiometry tells us what quantity of each reactant we need in order to get enough of our desired product.
What is stoichiometry in chemistry?
Chemical Stoichiometry refers to the quantitative study of the reactants and products involved in a chemical reaction. The word “ stoichiometry” is derived from the Greek word “stoikhein” meaning element and “metron” meaning measure.
How do you calculate the mass of a reactant?
- mass O2 = moles(O2) × molar mass(O2) (a) Calculate moles(Mg) = mass(Mg) ÷ molar mass(Mg) moles(Mg) = 12.2 ÷ 24.31 = 0.50 mol. …
- mass MgO = moles(MgO) × molar mass(MgO) (a) Calculate moles Mg. moles(Mg) = mass(Mg) ÷ molar mass(Mg)
What is a 1 mole?
A mole is defined as 6.02214076 × 1023 of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance.
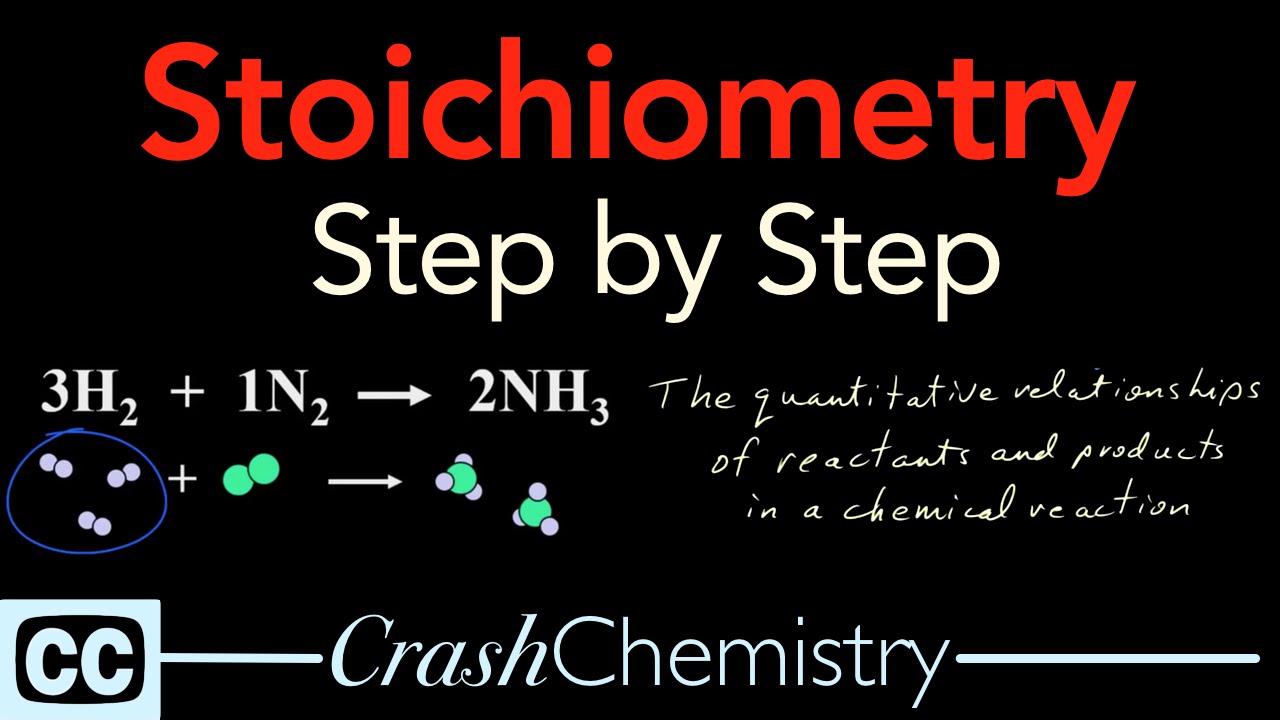
Stoichiometry Tutorial: Step by Step Video + review problems explained | Crash Chemistry Academy
Images related to the topicStoichiometry Tutorial: Step by Step Video + review problems explained | Crash Chemistry Academy
How much is a mole?
One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro’s number or Avogadro’s constant. The concept of the mole can be used to convert between mass and number of particles.. Created by Sal Khan.
How many grams are in a mole?
There are three steps to converting grams of a substance to moles: ▪ Step 1: Determine how many grams of a substance are in the problem. Use the periodic table to check the atomic mass, this is the number of grams per mole → 1 mole of Aluminum is 26.982 g ▪ Written as a fraction this is …
Related searches to How do you do Stoich?
- how do you do stoichiometry step by step
- how do you calculate stoichiometry?
- how do you do stoich in chemistry
- how to do stoichiometry ratios
- how do you do stoichiometry chemistry
- stoichiometry problems and answers
- how do you do stoichiometry for dummies
- how do you do mole to mole stoichiometry
- how do you do stoich problems
- what is the first rule of stoichiometry
- what is needed to perform a stoichiometry calculation
- how do you solve stoichiometry step by step
- stoichiometry problems and solutions pdf
- what is stoichiometry
- stoichiometry pdf
- how to do stoichiometry step by step
- stoichiometry notes
- stoichiometry khan academy
- how is pi calculated manually
- why does stoichiometry work
- how does a* work
- how do you do stoichiometry
- how do you calculate stoichiometry
- stoichiometry for dummies
- how do parameters work
Information related to the topic How do you do Stoich?
Here are the search results of the thread How do you do Stoich? from Bing. You can read more if you want.
You have just come across an article on the topic How do you do Stoich?. If you found this article useful, please share it. Thank you very much.