Are you looking for an answer to the topic “How do you find the de Broglie wavelength of a proton?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Multiplying the mass and speed, we obtain the momentum of the particle: p = mv = 2.7309245*10–24 kg·m/s . If we divide the Planck constant by the momentum, we will obtain the de Broglie wavelength: h/p = 6.6261*10–34 / 2.7309245*10–24 = 2.426*10–10 m .A proton and an electron have same de-Broglie wavelength which of them moves fast and which possesses more K.E. Justify your answer. is constant, then from equation (i), p = constant. It means the velocity of electron is greater than that of proton. Kinetic energy of electron is greater than that of proton.The de Broglie wavelength of a proton in a particle accelerator is 1.30×10−14 1.30 × 10 − 14 m.
- h= Planck’s constant(6.62607015×10−34 Js)
- Velocity of the electron, v =2×106 ms-1.
- Mass of electron, m =9.1×10–31 Kg.
- Planck’s Constant, h = 6.62607015×10−34 Js.
- = 6.62607015×10−34 /(2×106)(9.1×10–31 )
- λ = 0.364×109m.
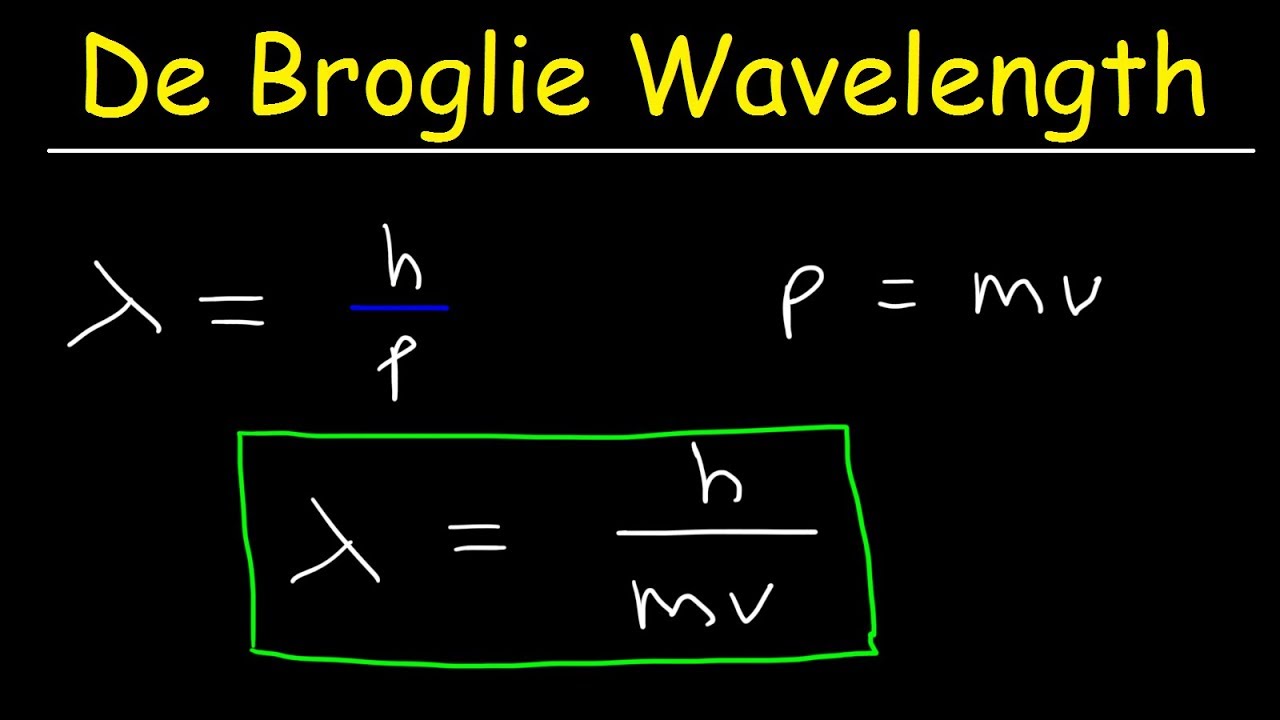
Table of Contents
Can a proton have a de Broglie wavelength?
A proton and an electron have same de-Broglie wavelength which of them moves fast and which possesses more K.E. Justify your answer. is constant, then from equation (i), p = constant. It means the velocity of electron is greater than that of proton. Kinetic energy of electron is greater than that of proton.
How do you find the de Broglie wavelength?
- h= Planck’s constant(6.62607015×10−34 Js)
- Velocity of the electron, v =2×106 ms-1.
- Mass of electron, m =9.1×10–31 Kg.
- Planck’s Constant, h = 6.62607015×10−34 Js.
- = 6.62607015×10−34 /(2×106)(9.1×10–31 )
- λ = 0.364×109m.
De Broglie Wavelength Problems In Chemistry
Images related to the topicDe Broglie Wavelength Problems In Chemistry

What is the de Broglie wavelength of a proton of energy?
The de Broglie wavelength of a proton in a particle accelerator is 1.30×10−14 1.30 × 10 − 14 m.
What is the de Broglie wavelength of proton and alpha particle?
Ratio of De-Broglie wavelengths of a proton and an alpha particle of the same energy is : 1 : 4. 4 : 1. 1 : 2.
Is a proton and electron have the same de Broglie wavelength then?
If a proton and electron have the same de broglie wavelength their momentum will be equal. Hence the correct answer is option (A) Momentum of electron = momentum of proton. The de broglie’s wave equation is only applicable for micro particles.
Does a proton have a wavelength?
The de Broglie wavelength of a particle is inversely proportional to its momentum p = m v; since a proton is about 1800 times more massive than an electron, its momentum at the same speed is 1800 times that of an electron, and therefore its wavelength 1800 times smaller.
What is de Broglie wavelength and its formula?
λ = h m v = h momentum : where ‘h’ is the Plank’s constant. This equation relating the momentum of a particle with its wavelength is de Broglie equation and the wavelength calculated using this relation is de Broglie wavelength.
See some more details on the topic How do you find the de Broglie wavelength of a proton? here:
The Wave Nature of Matter | Physics II
The de Broglie wavelength for massless particles was well established in the 1920s for photons, and it has since been observed that all massless particles have …
The de – Broglie wavelength of a proton accelerated by 400 V is
The de Broglie wavelength would be 1.43xx10^(-12)m I would approach the problem this way: First, the de Broglie wavelength is given by …
What is the formula of de Broglie equation?
λ = h/mv, where λ is wavelength, h is Planck’s constant, m is the mass of a particle, moving at a velocity v. de Broglie suggested that particles can exhibit properties of waves.
What is the de Broglie wavelength of an electron?
Applications of de Broglie Waves
10 eV electrons (which is the typical energy of an electron in an electron microscope): de Broglie wavelength = 3.9 x 10–10 m. This is comparable to the spacing between atoms.
What is the de Broglie wavelength of a neutron?
8/T Ao.
Does de Broglie equation apply to photons?
Yes, photons have a de Broglie wavelength, because photons have momentum associated with them when they are in motion even though they don’t have a rest mass.
De Broglie wavelength | Physics | Khan Academy
Images related to the topicDe Broglie wavelength | Physics | Khan Academy

What is the de Broglie wavelength of proton is accelerating with potential V is?
⇒λα=2 λ.
When proton and electron travels with same velocity the ratio of de Broglie wavelength is?
λe/λp = 1836:1.
What is the ratio of the de Broglie wavelengths proton and an α particle if they are accelerated by the same potential difference?
A proton and an alpha particle are accelerated through the same potential difference. The ratio of their de Broglie wavelengths (p) is: (a)1.
Is the de Broglie wavelength for a proton and alpha particle are equal then the ratio of their velocities will be?
If debroglie wavelength of proton and alpha particle are equal then ratio of their velocities are. m is mass and v is velocity. By equating momentum for Both we can easily find that velocity of Proton will be four times that of Alpha particle Because alpha particle is four times Heavies than that of Proton.
Which has more energy proton or electron?
A proton and an electron have same kinetic energy. Since the mass of proton is higher than electron, we can say proton has more energy than electron.
When the velocity of an electron increases its de Broglie wavelength?
So if the velocity of the electron increases, the de Broglie wavelength decreases.
Can a photon and an electron of the same momentum have the same wavelength compare their wavelengths of the two have the same energy?
The correct answer is linear momentum. The de-Broglie wavelength of a particle or a photon is given by λ = h/p where h is Planck’s constant and p is the momentum. As the electron and the photon are having the same wavelength λ, the momentum of both of them will be the same.
How do you find the speed of a proton?
The wavelength equation for de Broglie wavelength is used as there is only one variable. The speed of the proton is given as $ \dfrac{1}{{100}} $ of the speed of light in vacuum. This means that the speed of the proton is $ v = \dfrac{{3 \times {{10}^8}}}{{100}} = 3 \times {10^6}m/s $ .
What is the velocity of proton?
At these times, a typical proton density was 10 to 20 per cubic centimeter, with most protons having velocities between 400 and 650 kilometers per second.
How do you find the wavelength of a particle?
He postulated that a wavelength could be associated with a particle once you know its mass and its velocity. The deBroglie wavelength is defined as follows: lambda = h/mv , where the greek letter lambda represents the wavelength, h is Planck’s contant, m is the particle’s mass and v is its velocity.
A proton and an alpha particle have same de-Broglie wavelength determine the ratio of (i) their ac.
Images related to the topicA proton and an alpha particle have same de-Broglie wavelength determine the ratio of (i) their ac.

How do you find the wavelength of an electron?
wavelength of an electron is calculated for a given energy (accelerating voltage) by using the de Broglie relation between the momentum p and the wavelength λ of an electron (λ=h/p, h is Planck constant).
How can wavelength be calculated?
- Use a photometer to measure the energy of a wave.
- Convert the energy into joules (J).
- Divide the energy by Planck’s constant, 6.626 x 10–34, to get the frequency of the wave.
- Divide the speed of light, ~300,000,000 m/s, by the frequency to get wavelength.
Related searches to How do you find the de Broglie wavelength of a proton?
- de broglie wavelength of neutron
- wavelength of a proton calculator
- mass of proton
- de broglie wavelength of electron
- de broglie wavelength formula
- de broglie wavelength of proton and electron
- velocity of electron with de broglie wavelength
- de broglie wavelength of photon
- how do you find the de broglie wavelength
Information related to the topic How do you find the de Broglie wavelength of a proton?
Here are the search results of the thread How do you find the de Broglie wavelength of a proton? from Bing. You can read more if you want.
You have just come across an article on the topic How do you find the de Broglie wavelength of a proton?. If you found this article useful, please share it. Thank you very much.