Are you looking for an answer to the topic “How do you find the de Broglie wavelength of an electron from kinetic energy?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
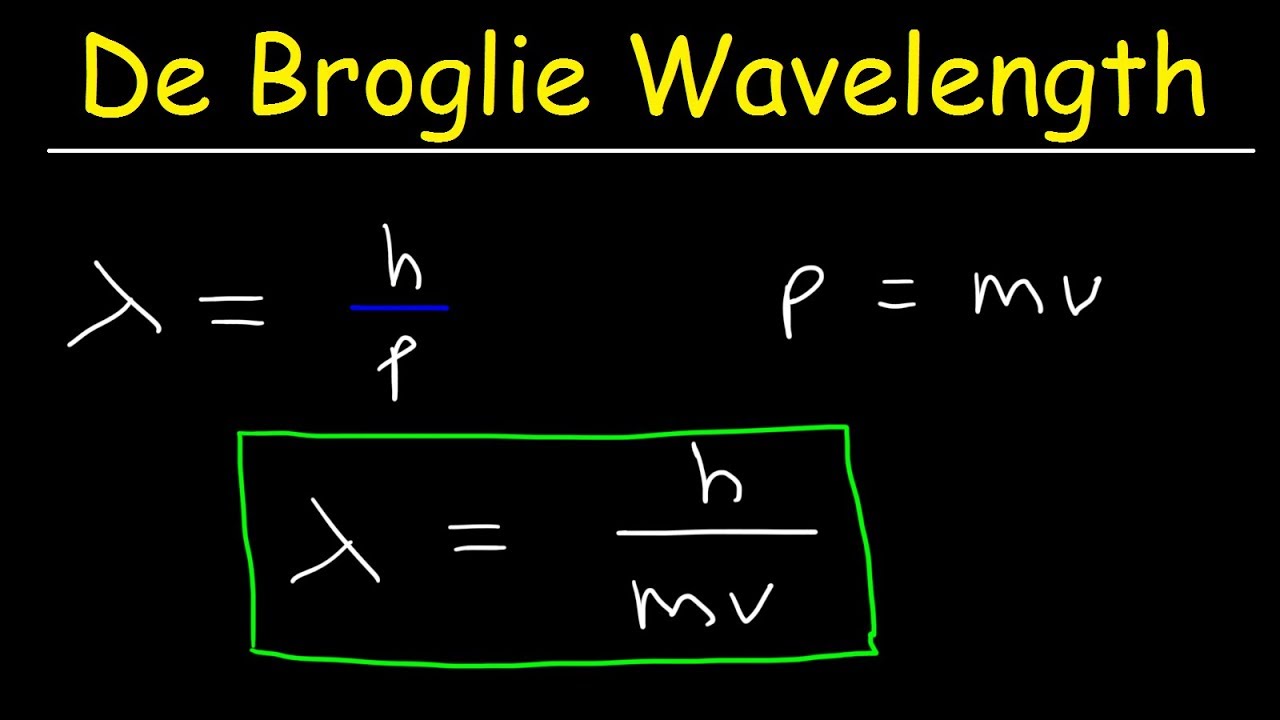
If you double the kinetic energy of a particle, how does the deBroglie wavelength change? Solution: λ = h/p, E = p2/(2m), p is proportional to √E, l is proportional to 1/√E. λ2/λ1 = √(E1/E2) = 1/√2.De-Broglie wavelength of a particle is inversely proportional to the momentum of that particular body. We should know that kinetic energy and momentum of a particle is related as. E=P22m.Equation Number Two: λ = h/p
it is here in case you migt be interested in it. Suppose an electron has momentum equal to p, then its wavelength is λ = h/p and its frequency is f = E/h.

Table of Contents
How do you find de Broglie wavelength from kinetic energy?
De-Broglie wavelength of a particle is inversely proportional to the momentum of that particular body. We should know that kinetic energy and momentum of a particle is related as. E=P22m.
How do you find the wavelength of an electron given kinetic energy?
Equation Number Two: λ = h/p
it is here in case you migt be interested in it. Suppose an electron has momentum equal to p, then its wavelength is λ = h/p and its frequency is f = E/h.
Calculate the de-Broglie wavelength of an electron of kinetic energy 100 eV
Images related to the topicCalculate the de-Broglie wavelength of an electron of kinetic energy 100 eV

What is the de Broglie wavelength of an electron with a kinetic energy?
Solution : `lambda=(h)/(sqrt(2mE))` <br> `=(6.625 xx 10^(-34))/(sqrt(2 xx 9.1 xx 10^(31) xx 120 xx 1.6 xx 10^(-19)))` <br> `=1.121 xx 10^(-10)`m. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
What is the de Broglie wavelength of an electron with kinetic energy 500ev?
E = hc/λ or λ = hc/E = (6.626 x 10-34 Js)(2.99 x 108 m/s)/(8.1 x 10-17 J) λ = 2.44 x 10-9 m = 2.44 nm, which is about 5 times too big!
What is the de Broglie wavelength of an electron?
Applications of de Broglie Waves
10 eV electrons (which is the typical energy of an electron in an electron microscope): de Broglie wavelength = 3.9 x 10–10 m. This is comparable to the spacing between atoms.
What is the wavelength of an electron that has 2.0 Kev of kinetic energy?
m = 0.0275 nm.
What is the de Broglie wavelength of an electron with a kinetic energy of 120 eV?
Therefore, the de Broglie wavelength of the electron is 0.112 nm.
See some more details on the topic How do you find the de Broglie wavelength of an electron from kinetic energy? here:
The deBroglie Equation: Relating a Particle’s Energy to its …
Suppose an electron has momentum equal to p, then its wavelength is λ = h/p and its frequency is f = E/h. (ChemTeam comment: note the use of the two de Broglie …
What is the de Broglie wavelength of an electron of kinetic …
The electron has kinetic energy E = (mv^2) /2 where m is the mass and v is the velocity of the electron. It’s momentum is p = mv = √( 2 m E) Thus the de …
DeBroglie Wavelength – Hyperphysics
For an electron with KE = 1 eV and rest mass energy 0.511 MeV, the associated DeBroglie wavelength is 1.23 nm, about a thousand times smaller than a 1 eV …
Calculate kinetic energy of an electron having wavelength …
Hint: We will use the equation to find de-Broglie wavelength to find the kinetic energy of the electron with wavelength 1nm. De-Broglie wavelength of a …
What is the de Broglie wavelength of an electron with kinetic energy 100ev?
=1.23 × 10−10 m = 1. 23Ao.
What is the de Broglie wavelength of an electron having Ke of 5ev?
So, the de broglie wavelength associated with the electron is 5.47 A.
Calculation of de Broglie wavelength of the electron
Images related to the topicCalculation of de Broglie wavelength of the electron

Which of the following is the de Broglie wavelength of an electron with a kinetic energy of 1.00 eV?
For an electron with KE = 1 eV and rest mass energy 0.511 MeV, the associated DeBroglie wavelength is 1.23 nm, about a thousand times smaller than a 1 eV photon.
What is the de Broglie wavelength of an electron that has 200 kev of kinetic energy?
λ=0.388 nm .
What is de Broglie wavelength of an electron whose kinetic energy is 1 eV?
112×10−9 m=112 pm.
What is described by de Broglie equation?
The de Broglie equation is an equation used to describe the wave properties of matter, specifically, the wave nature of the electron: λ = h/mv, where λ is wavelength, h is Planck’s constant, m is the mass of a particle, moving at a velocity v. de Broglie suggested that particles can exhibit properties of waves.
What is de Broglie’s theory?
De Broglie’s hypothesis of matter waves postulates that any particle of matter that has linear momentum is also a wave. The wavelength of a matter wave associated with a particle is inversely proportional to the magnitude of the particle’s linear momentum.
What happens to the de Broglie wavelength of an electron if its momentum is doubled?
Detailed Solution. This implies that de-Broglie wavelength is inversely proportional to momentum. Therefore, if the momentum is doubled, the de-Broglie wavelength becomes half.
What is de Broglie wavelength for an electron with velocity of light?
The de Broglie wavelength of an electron moving with a velocity c/2 (c=velocity of light in vacuum) is equal to the wavelength of a photon.
What is the de Broglie wavelength of electron having energy 10k EV?
Therefore, wavelength of given electron will be 1.2 A°.
De Broglie Wavelength Problems In Chemistry
Images related to the topicDe Broglie Wavelength Problems In Chemistry
What is the de Broglie wavelength of an electron accelerated?
λ = 1.23√V nm.
What is de Broglie wavelength of a non relativistic proton with a kinetic energy of 1.0 eV?
λ = h p = h c K ( K + 2 E 0 ) = 1.241 eV · μ m ( 1.0 eV ) [ 1.0 eV+ 2 ( 511 keV ) ] = 1.23 nm .
Related searches to How do you find the de Broglie wavelength of an electron from kinetic energy?
- electron wavelength calculator
- wavelength of electron formula
- electron energy to wavelength calculator
- de broglie wavelength equation
- de broglie wavelength of proton
- de broglie wavelength calculator
- what is the de broglie wavelength of an electron
- de broglie equation
Information related to the topic How do you find the de Broglie wavelength of an electron from kinetic energy?
Here are the search results of the thread How do you find the de Broglie wavelength of an electron from kinetic energy? from Bing. You can read more if you want.
You have just come across an article on the topic How do you find the de Broglie wavelength of an electron from kinetic energy?. If you found this article useful, please share it. Thank you very much.