Are you looking for an answer to the topic “How do you find the density of an aniline?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Keep Reading
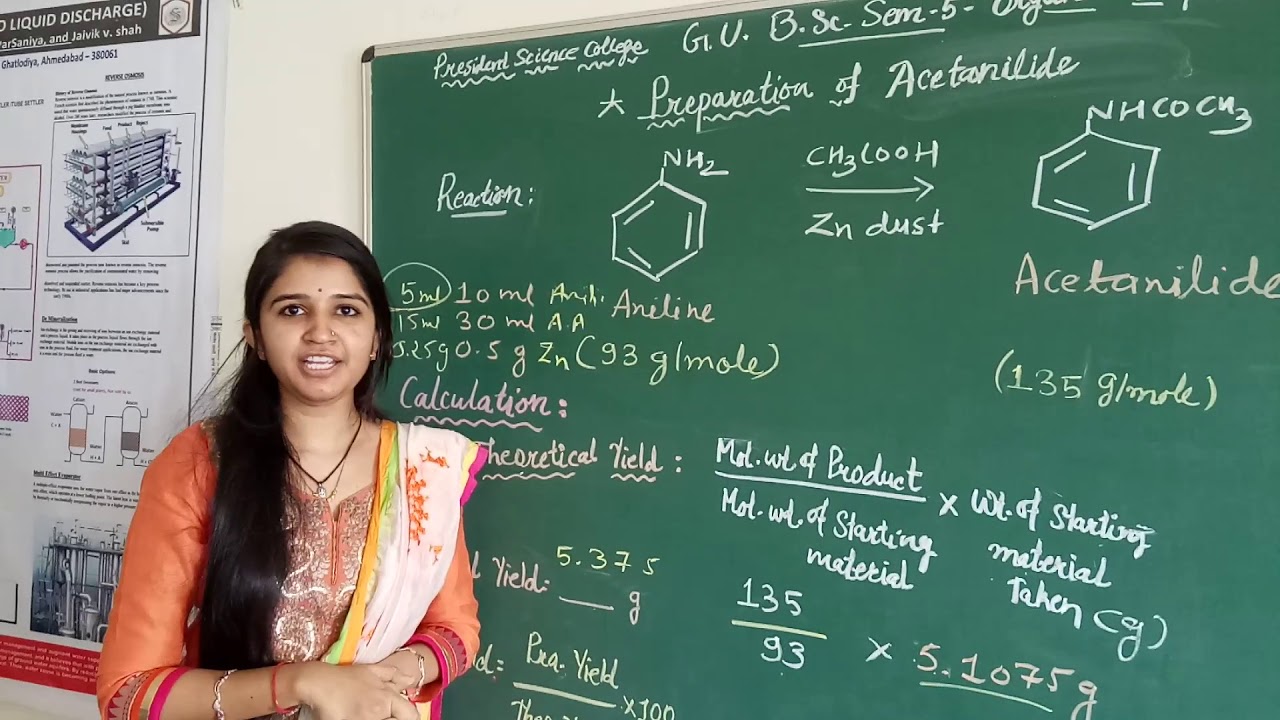
Table of Contents
What is the density of aniline?
What is the density of aniline g mL?
1.022 g/mL at 25 °C (lit.)
Organic Preparation: Acetanilide from aniline (By, Dr.Jaivik SIr Miss Apeksha)
Images related to the topicOrganic Preparation: Acetanilide from aniline (By, Dr.Jaivik SIr Miss Apeksha)
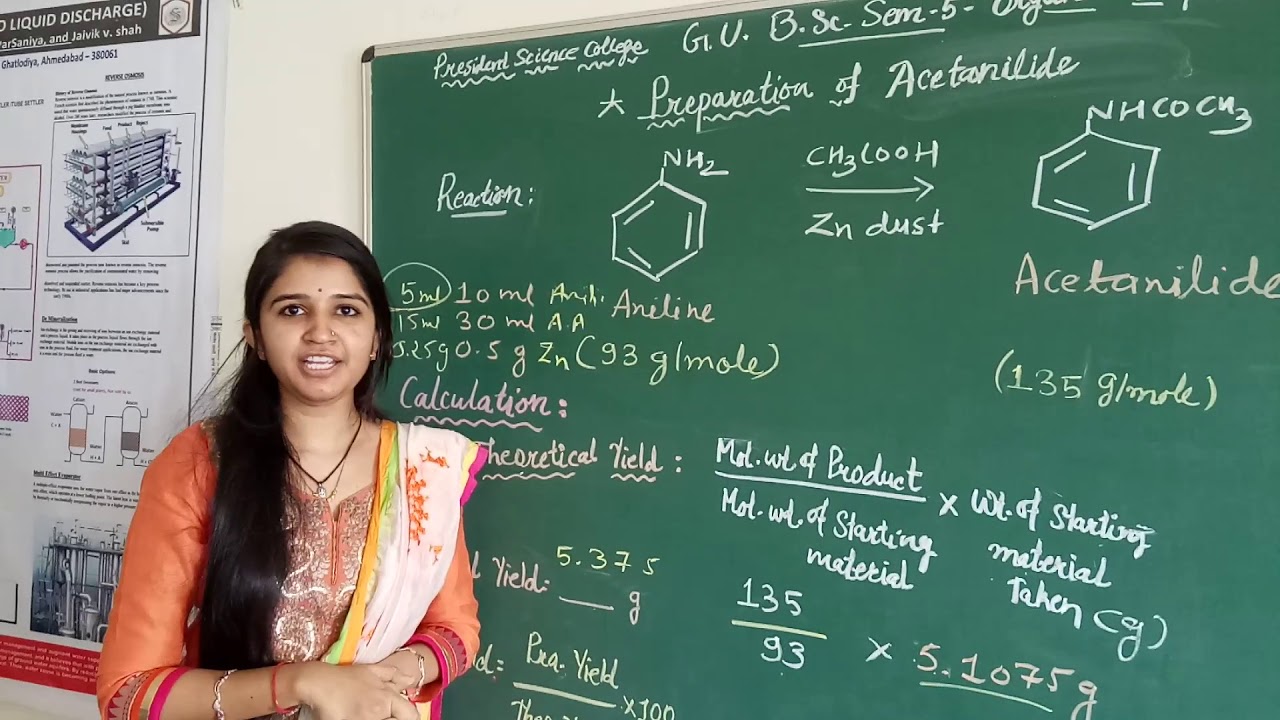
How do you find the molecular weight of an aniline?
What is the density of Acetanilide?
What is the formula of aniline?
How is aniline estimated?
Assertion – Aniline can be estimated by treatment with excess of of bromine water solution. <br> Reason – Aniline reacts quantiatively with excess of bromine water solution forming insoluble, 2,4,6-tribromoaniline.
What is aniline derivative?
Aniline derivatives (occasionally referred to as coal tar analgesics) are strong antipyretics that may exert their antipyretic effects on the thermoregulatory center of the hypothalamus.
See some more details on the topic How do you find the density of an aniline? here:
Density of Aniline in 285 units and reference information
Aniline weighs 1 023.5 kg/m³ (63.89502 lb/ft³) · Density of Aniline in a few select units of density measurement: · Density of Aniline g cm3 = 1.02 g/cm³ · Density …
Aniline | C6H5NH2 – PubChem
Aniline appears as a yellowish to brownish oily liquid with a musty fishy odor. Melting point -6°C; boiling point 184°C; flash point 158°F. Denser than …
62-53-3(Aniline) Product Description – ChemicalBook
1.022 g/mL at 25 °C (lit.) vapor density : 3.22 (185 °C, vs air) …
Aniline, 99.0 C6H5NH2 – Sigma-Aldrich
refractive index. n20/D 1.586 (lit.) bp. 184 °C (lit.) mp. −6 °C (lit.) density. 1.022 g/mL at 25 °C (lit.) …
What is the difference between amines and aniline?
As nouns the difference between amine and aniline is that amine is (inorganic chemistry) a functional group formally derived from ammonia by replacing one, two or three hydrogen atoms with hydrocarbon or other radicals while aniline is (organic compound) the simplest aromatic amine, c6h5nh2, synthesized by the …
Is aniline solid or liquid?
Aniline is a colourless to brown, oily liquid which darkens on exposure to air and light. It has a characteristic amine odour (detectable at 0.6 to 10 ppm ) and burning taste. Hygroscopic. Moderately soluble in water.
What is the equivalent weight of aniline?
The equivalent weight and molecular weight of Aniline are same. Explanation: Aniline is a primary aryl amine a functional group of amine family. Aniline is a primary aryl amine group that has molecular weight of 93.3g.
Is aniline a strong base?
A primary aromatic amine, aniline is a weak base and forms salts with mineral acids.
What is the functional group of aniline?
Aniline is a primary arylamine in which an amino functional group is substituted for one of the benzene hydrogens. It is a primary arylamine and a member of anilines.
Resonance Structures of Aniline
Images related to the topicResonance Structures of Aniline

How do you convert aniline to acetanilide?
Dissolve aniline in hydrochloric acid and add acetic anhydride stir well. Pour the mixture to sodium acetate in water. Acetanilide is formed which can be separated and recrystallised by ethyl alcohol.
What is the formula of acetanilide?
What is the volume of acetanilide?
| ACD/LogP: | 1.08 |
|---|---|
| Polar Surface Area: | 29 Å2 |
| Polarizability: | 16.1±0.5 10–24cm3 |
| Surface Tension: | 41.5±3.0 dyne/cm |
| Molar Volume: | 122.5±3.0 cm3 |
What is an aniline in chemistry?
Aniline, also known as aminobenzene or phenylamine, has 6 carbon (C) atoms, 7 hydrogen (H) atoms, and 1 nitrogen (N) atom in its chemical formula of C6H7N or C6H5NH2. Because aniline has an amino group in its structure, it is also an amine, hence it is classified as an aromatic amine.
How do you name aniline?
How do you pronounce aniline?
Break ‘aniline’ down into sounds: [AN] + [UH] + [LEEN] – say it out loud and exaggerate the sounds until you can consistently produce them.
Which reagent is used for estimation of aniline?
The best (among the studied) immobilized reagent for determining aniline was 1-naphthol. Xerogel modified with 1-naphthol was used for determining aniline in solutions by solid-phase spectrophotometry and with visual detection.
How will you prepare 0.1 N Brominating solution?
Brominating mixture solution (0.1 N) was prepared by dissolving 0.695 g of potassium bromate and 3.75 g of potassium bromide in 100 ml. distilled water. This solution is suitably diluted to give 0.02 M solution.
Which solution is taken in burette during estimation of phenol by Brominating method?
3. Titration with standard phenol solution: Pipette out 25 cm3 of standard phenol solution in a 250 cm3 conical flask and add 25 cm3 of distilled water and 5 cm3 concentrated HCI. Brominating mixture (taken in burette) is now added to this solution till it achieves light yellow colour and then add 5 cm3 of KI solution.
Why Does hair dye cause allergies?
Why some people are sensitive to hair dye
Many permanent and some semi-permanent hair dyes contain a chemical called paraphenylenediamine (PPD), which is a known irritant and allergen. Darker coloured dyes contain higher level of PPD. PPD is the cause of most reactions to hair dye.
Basicity of Aniline its Derivatives || Kota Ke Koncepts
Images related to the topicBasicity of Aniline its Derivatives || Kota Ke Koncepts

Why is aniline basic?
Basicity of Aniline Definition
The basic nature of aniline is due to the presence of amino group N H 2 {\rm{N}}{{\rm{H}}_{\rm{2}}} NH2, which has one lone pair of electrons on the nitrogen atom. Aniline, on reaction with a stronger acid, forms anilinism ion.
Why is aniline a weak base?
Aniline only reluctantly accepts a proton to form the anilinium ion, and hence is a weak base. When an -NH group is attached to an aliphatic radical it receives no comparable delocalization stabilization. It is less reluctant to accept a proton on its nitrogen lone pair, and hence aliphatic amines are stronger bases.
Related searches to How do you find the density of an aniline?
- aniline density gml
- how do you find the density of an aniline in water
- aniline solubility in water
- how do you find the density of an aniline solution
- density of aniline
- aniline melting point
- molecular weight of aniline
- aniline formula
- boiling point of aniline
- how do you find the density of an aniline at room temperature
- aniline structure
- how do you find the density of an aniline lab report
- aniline density g/ml
Information related to the topic How do you find the density of an aniline?
Here are the search results of the thread How do you find the density of an aniline? from Bing. You can read more if you want.
You have just come across an article on the topic How do you find the density of an aniline?. If you found this article useful, please share it. Thank you very much.