Are you looking for an answer to the topic “How do you find the slowest step in a mechanism?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Rate determining step is the slowest step within a chemical reaction. The slowest step determines the rate of chemical reaction. The slowest step of a chemical reaction can be determined by setting up a reaction mechanisms. Many reactions do not occur in a single reaction but they happen in multiple elementary steps.The slowest step in a reaction mechanism is known as the rate-determining step. The rate-determining step limits the overall rate and therefore determines the rate law for the overall reaction.
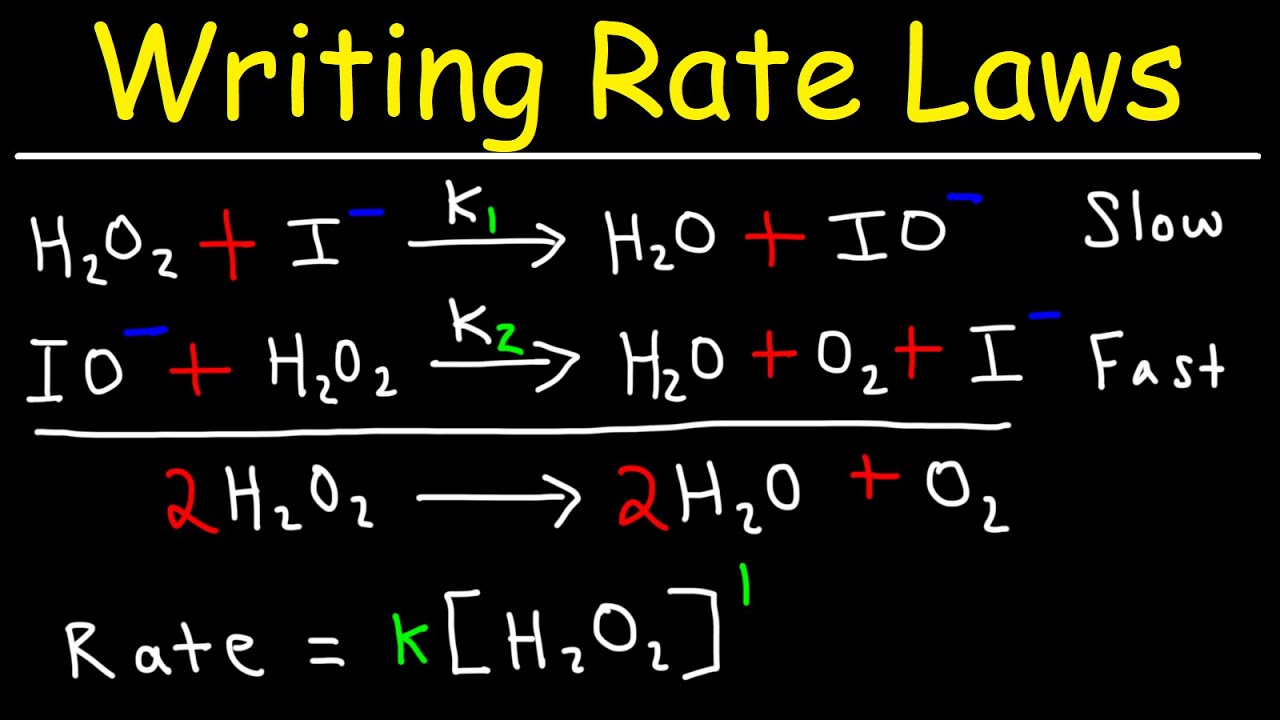
Which is the slowest step in a reaction mechanism?
The slowest step in a reaction mechanism is known as the rate-determining step. The rate-determining step limits the overall rate and therefore determines the rate law for the overall reaction.
Why is the slowest step the rate determining step?
Rate-determining steps are slow because of how the mechanism works, which is just another way of saying “it is what it is”, and isn’t much of an answer.
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step – Chemical Kinetics
[su_youtube url=”https://www.youtube.com/watch?v=B1bWIrOe0SE”]
Images related to the topicWriting Rate Laws of Reaction Mechanisms Using The Rate Determining Step – Chemical Kinetics

Which statement about the slow step in the mechanism for a reaction is true?
The answer is option C) It controls the rate at which the products are produced. The slowest step in a multi-step reaction is entirely…
How do you find the rate law using a mechanism?
A The rate law for step 1 is rate = k1[NO2]2; for step 2, it is rate = k2[N2O4][CO]. B If step 1 is slow (and therefore the rate-determining step), then the overall rate law for the reaction will be the same: rate = k1[NO2]2. This is the same as the experimentally determined rate law.
Which step is the rate determining step in the mechanism of electrophilic substitution reaction?
Which of the following is rate determining step in electrophilic substitution reaction? Explanation: Attack by an electrophilic reagent on benzene ring is a rate determining step in electrophilic substitution reaction. It is also the slowest step of the reaction.
Which step in sn1 reaction is a slow rate determining step?
The formation of a carbocation is the slow, or rate-determining, step. The subsequent step, formation of a bond between the nucleophile and the carbocation, occurs very rapidly. Because the slow step of the reaction involves only the substrate, the reaction is unimolecular.
Determine Rate Law from Reaction Mechanisms, Fast then Slow Step: Part I
[su_youtube url=”https://www.youtube.com/watch?v=TtViElZcIg8″]
Images related to the topicDetermine Rate Law from Reaction Mechanisms, Fast then Slow Step: Part I

See some more details on the topic How do you find the slowest step in a mechanism? here:
Reaction mechanism and rate law (article) | Khan Academy
The slowest step in a reaction mechanism is known as the rate-determining step. The rate-determining step limits the overall rate and therefore determines the …
Determining Slow Step – CHEMISTRY COMMUNITY
The two important components to look for when determining reaction mechanisms are 1) that the sum of elementary steps = overall reaction and 2) …
Rate-determining step – Wikipedia
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS) or …
Reaction Mechanisms | Boundless Chemistry | Course Hero
The rate-determining step is the slowest step in a reaction mechanism. Because it is the slowest, it determines the rate of the overall reaction.
What is the slow and rate determining stage of electrophilic?
In the first, slow or rate-determining, step the electrophile forms a sigma-bond to the benzene ring, generating a positively charged benzenonium intermediate. In the second, fast step, a proton is removed from this intermediate, yielding a substituted benzene ring.
Which of the following step is the rate determining step of contact theory?
7. Which of the following step is the rate determining step of contact theory? Explanation: Chemical reaction at the surface is the slowest and rate determining step of contact theory.
What is the molecularity of the rate determining step in the proposed mechanism?
The molecularity of a reaction is defined as the number of molecules or ions that participate in the rate determining step. A mechanism in which two reacting species combine in the transition state of the rate-determining step is called bimolecular.
How many elementary steps are in the reaction mechanism?
The equation in an elementary step represents the reaction at the molecular level, not the overall reaction. Based on numbers of molecules involved in the elementary step, there are three kinds of elementary steps: unimolecular step (or process), bimolecular process, and trimolecular process.
Reaction mechanism and rate law | Kinetics | AP Chemistry | Khan Academy
[su_youtube url=”https://www.youtube.com/watch?v=ShzW1LoQgoc”]
Images related to the topicReaction mechanism and rate law | Kinetics | AP Chemistry | Khan Academy

How do you find the mechanism of a reaction?
The overall sequence of elementary reactions is the mechanism of the reaction. The sum of the individual steps, or elementary reactions, in the mechanism must give the balanced chemical equation for the overall reaction. The overall sequence of elementary reactions is the mechanism of the reaction.
What is the rate determining step for this reaction quizlet?
What is the rate-determining step? The slowest step in a reaction, which dictates how quickly a reaction can proceed.
Related searches to How do you find the slowest step in a mechanism?
- what is the rate law predicted by this mechanism
- how do you find the slowest step in a reaction
- rate determining step example
- why is the slowest step the rate determining step
- reaction mechanism examples with answers
- how to determine rate determining step from graph
- determine the rate law for the overall reaction with an intermediate
- reaction mechanism
- how to determine slowest step in a mechanism
- does the rate determining step have the highest activation energy
Information related to the topic How do you find the slowest step in a mechanism?
Here are the search results of the thread How do you find the slowest step in a mechanism? from Bing. You can read more if you want.
You have just come across an article on the topic How do you find the slowest step in a mechanism?. If you found this article useful, please share it. Thank you very much.
Leave a Reply