Are you looking for an answer to the topic “How do you know which isotope is more abundant?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
To determine the most abundant isotopic form of an element, compare given isotopes to the weighted average on the periodic table. For example, the three hydrogen isotopes (shown above) are H-1, H-2, and H-3. The atomic mass or weighted average of hydrogen is around 1.008 amu ( look again at the periodic table).To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.Summary. Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Isotopes have different atomic masses. The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element.

Table of Contents
How do you find the abundance of an isotope?
To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.
What makes an abundant isotope?
Summary. Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Isotopes have different atomic masses. The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element.
How to determine which isotope is the most abundant
Images related to the topicHow to determine which isotope is the most abundant

Is the heavier isotope more abundant?
In isotope science, a “heavy isotope” is the stable isotope or stable isotopes of an element, which are heavier than the most abundant isotope.
How do you determine abundance?
Abundance is in simplest terms usually measured by identifying and counting every individual of every species in a given sector. It is common for the distribution of species to be skewed so that a few species take up the bulk of individuals collected.
Why is one isotope more abundant than another?
Although isotopes of the same element are twins when it comes to reactivity, the different number of neutrons means that they have a different mass. Certain isotopes are more abundant in some materials than others since some physical and chemical processes “prefer” one isotope over another.
Which is the more abundant isotope CL 35 or CL 37?
This is because the chlorine-35 isotope is much more abundant than the chlorine-37 isotope. The table shows the mass numbers and abundances of naturally-occurring copper isotopes.
Is N 14 or N 15 more abundant?
Natural nitrogen (7N) consists of two stable isotopes: the vast majority (99.6%) of naturally occurring nitrogen is nitrogen-14, with the remainder being nitrogen-15.
See some more details on the topic How do you know which isotope is more abundant? here:
Isotopes and mass spectrometry (article) | Khan Academy
Find out how isotopes can be detected using mass spectrometry. … which means that some isotopes are more naturally abundant on Earth than others.
How to Calculate the Percent Abundance of an Isotope
Step 1: Find the Average Atomic Mass · Step 2: Set Up the Relative Abundance Problem · Step 3: Solve for x to Get the Relative Abundance of the …
Isotope Abundance and Average Atomic Mass | ChemTalk
When looking at the periodic table, each element has a value displayed for the atomic mass. If you look closely, it is clear that these values …
Which isotope has the highest isotopic abundance?
The most abundant isotope is Si-28 which accounts for 92.23% of naturally occurring silicon.
How do you find the percent abundance of an isotope mass?
Step 1: List the known and unknown quantities and plan the problem. Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
Is Cu 65 or Cu 63 more abundant?
Copper: Physiology
Cu has 29 isotopes, two stable isotopes (63Cu and 65Cu), and 27 radioisotopes. The most abundant isotope is 63Cu that accounts for approximately 69% of naturally occurring Cu.
How to Find the Abundance of Each Isotope
Images related to the topicHow to Find the Abundance of Each Isotope

What is the most abundant isotope of hydrogen?
Protium, 1H, has no neutrons in its nucleus and is the most common form of hydrogen, with an atomic mass of ~1.0078 Da (dalton) and an isotopic abundance of ~99.972% of all hydrogen on Earth.
Are lighter isotopes more abundant?
The lighter isotope (lower mass number) usually dominates in natural abundance, with one or more heavier isotopes existing in less than a few percent.
Which isotope is more abundant on earth boron 10 or boron 11?
Answer and Explanation: Boron-11 is more abundant. Boron is identified as atoms containing five protons in the nucleus. This means that boron-10 would have five neutrons in…
How do you describe relative abundance?
Species abundance is the number of individuals per species, and relative abundance refers to the evenness of distribution of individuals among species in a community.
What do rank abundance curves show?
A rank abundance curve or Whittaker plot is a chart used by ecologists to display relative species abundance, a component of biodiversity. It can also be used to visualize species richness and species evenness.
How do you calculate relative richness and species abundance?
You can calculate species relative abundance byTotal Number of Individual species (Isi) divided by Total Number of Species Population ( ∑ Nsi) multiply by one hundred (100).
Are more stable isotopes more abundant?
In contrast, radioactive isotopes (e.g., 14C) are unstable and will decay into other elements. The less abundant stable isotope(s) of an element have one or two additional neutrons than protons, and thus are heavier than the more common stable isotope for those elements.
Why is carbon-12 the most abundant?
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon (carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars.
How do you find the percent abundance of three isotopes?
Calculate the average atomic mass using the atomic masses of each isotope and their percent abundances. Divide each percent abundance by 100 to convert it to decimal form. Multiply this value by the isotope’s atomic mass.
How To Find The Percent Abundance of Each Isotope – Chemistry
Images related to the topicHow To Find The Percent Abundance of Each Isotope – Chemistry
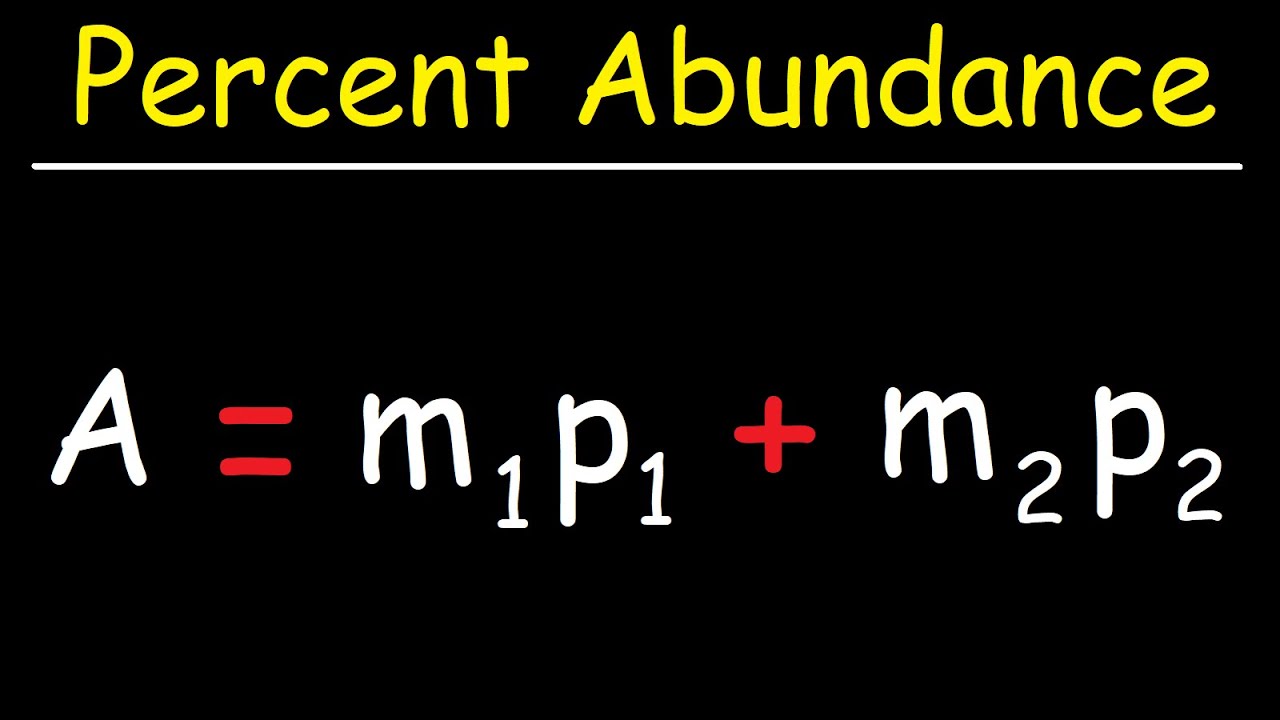
Which isotopes in chlorine is more abundant?
Explanation: As you may know, chlorine’s relative atomic mass is 35.5, but it has two significantly occurring isotopes: chlorine-35 and chlorine-37. From this information we can determine that chlorine-35 must be the most common of the two, and so is chlorine’s most common isotope.
Which isotope of chlorine would you expect to be more stable chlorine-37 or chlorine-35?
| Isotope | ||
|---|---|---|
| abundance | half-life (t1/2) | |
| 35Cl | 76% | stable |
| 36Cl | trace | 3.01×105 y |
| 37Cl | 24% | stable |
Related searches to How do you know which isotope is more abundant?
- how do you know which isotope is more abundant
- most abundant isotope meaning
- which hydrogen isotope is the most abundant
- what is an isotope
- how to calculate abundance of isotopes
- which isotope is more abundant cl-35 or cl-37
- which isotope is more abundant boron-10 or boron-11
- what elements most common isotope has 5 neutrons
- which isotope is more abundant lithium 6 and lithium 7
- which isotope is more abundant n14 or n15
- which isotope is more abundant boron 10 or boron 11
- which isotope is more abundant cl 35 or cl 37
- how to find which isotope is the most abundant
- how do you find out which isotope is more abundant
Information related to the topic How do you know which isotope is more abundant?
Here are the search results of the thread How do you know which isotope is more abundant? from Bing. You can read more if you want.
You have just come across an article on the topic How do you know which isotope is more abundant?. If you found this article useful, please share it. Thank you very much.