Are you looking for an answer to the topic “How do you label carbons?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Carbon atoms are numbered beginning from the reactive end of the molecule, the CHO (aldehyde) or “C” double bonded “O” (carbonyl) end of the molecule. Each carbon atom is then numbered in order through the end of the chain.
…
| Number of Carbons | Name |
|---|---|
| 1 | methane |
| 2 | ethane |
| 3 | propane |
| 4 | butane |

Table of Contents
How do you name carbons?
…
| Number of Carbons | Name |
|---|---|
| 1 | methane |
| 2 | ethane |
| 3 | propane |
| 4 | butane |
Do you number carbons left to right or right to left?
It is important to number the molecule from the correct end (in other words, in this example do you number the alkane from right to left or left to right). Following this rule, on this molecule you number from right to left, as the 2-carbon substituent is closer to that end. 3.
Practice Problems: Labeling Carbons
Images related to the topicPractice Problems: Labeling Carbons

What is the prefix for a 2 carbon chain?
| Number of carbon atoms in the parent chain | Root word |
|---|---|
| 2 | Eth |
| 3 | Prop |
| 4 | But |
| 5 | Pent |
What is CH and ch2?
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon atom, which is connected to the remainder of the molecule by two single bonds. The group may be represented as CH2<, where the ‘<‘ denotes the two bonds.
What is a 4 carbon chain called?
Alkanes with unbranched carbon chains are simply named by the number of carbons in the chain. The first four members of the series (in terms of number of carbon atoms) are named as follows: CH4 = methane = one hydrogen-saturated carbon.
How will you name a hydrocarbon?
1. The first part of the name is based on the length of the longest carbon chain in the molecule. 2. The end of the name is given by the number of bonds between carbon atoms.
What is a 5 carbon ring called?
Compounds containing 5 or 6 carbons are called cyclic.
See some more details on the topic How do you label carbons? here:
Organic Nomenclature – Number Carbons – Chemistry …
In order to be able to precisely define the position of these functional groups and also the carbon branches, a number is assigned to each carbon. The numbers …
Displaying the Label of Carbon Atoms | Chemaxon Docs
Displaying the Label of Carbon Atoms. It is possible to change the carbon atom display options on the Structure tab after navigating to Edit > Preferences.
How to name organic compounds using the IUPAC rules
In summary, the name of the compound is written out with the substituents in alphabetical order followed by the base name (derived from the number of carbons in …
Carbon label – Wikipedia
Carbon label is a form of isotopic labeling where a carbon-12 atom has been replaced with either a carbon-13 atom or a carbon-14 atom in a chemical compound …
How do you name alkanes?
- The locant: The number indicating where the substituent is.
- The prefix: The substituent attached to the alkane. Ends with -yl.
- The Parent: The alkane parent chain. Ends with -ane.
- Suffix: The functional group attached to the alkane. Not always present.
IUPAC Nomenclature of Alkanes – Naming Organic Compounds
Images related to the topicIUPAC Nomenclature of Alkanes – Naming Organic Compounds

What part of each name shows the number of carbon atoms?
Each alkane has a characteristic, systematic name depending on the number of carbon atoms in the molecule. These names consist of a stem that indicates the number of carbon atoms in the chain plus the ending –ane. The stem meth– means one carbon atom, so methane is an alkane with one carbon atom.
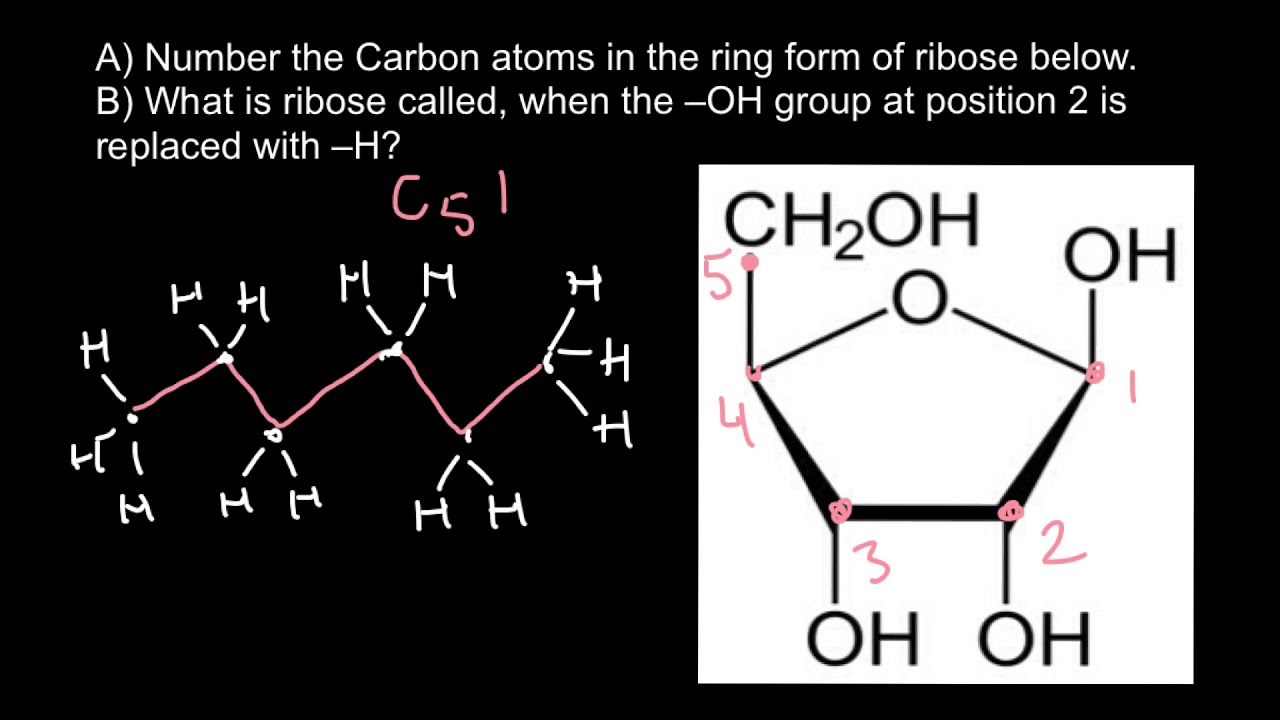
How are carbons numbered in sugars?
Numbering of Carbon Atoms
Carbon atoms are numbered beginning from the reactive end of the molecule, the CHO (aldehyde) or “C” double bonded “O” (carbonyl) end of the molecule. Each carbon atom is then numbered in order through the end of the chain.
What is the symbol for carbon on the periodic table?
carbon (C), nonmetallic chemical element in Group 14 (IVa) of the periodic table.
What is the prefix for 1 carbon?
| Prefix | Number of Carbon atoms | Formula |
|---|---|---|
| meth- | 1 | C |
| eth- | 2 | C2 |
| prop- | 3 | C3 |
| but- | 4 | C4 |
What is the name of an 11 carbon chain?
| Table 1. Summary of the Straight-Chain Alkanes | ||
|---|---|---|
| Name | Formula | Number of Structural Isomers |
| Decane | C10H22 | 75 |
| Undecane | C11H24 | |
| Dodecane | C12H26 |
What do prefixes (+) and before an organic compound mean?
Solution : Prefixes (+),(-) and `(+-)` imply dextrorotatory, laevorotatory and racemic modification respectively. Answer.
What is a CH group called?
In chemistry, a methine group or methine bridge is a trivalent functional group =CH−, derived formally from methane. It consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen.
How to number carbon atoms
Images related to the topicHow to number carbon atoms
What is CH3 CH CH CH3?
1,2-dimethylethene.
What is the IUPAC name for CH3 CH2 CH2 CH2 CH CH CH2 CH3?
All are single bonds without the presence of any functional groups so the IUPAC name is heptane.
Related searches to How do you label carbons?
- how do you number carbons in a ring
- how to number carbons in a nucleotide
- how to count carbons and hydrogens in organic chemistry
- how do you label carbons in dna
- how do you label carbons on the periodic table
- how do you label carbons in the body
- how do you label carbons in a ring
- how to number carbons
- carbon labeled periodic table
- how to count carbon atoms in a structure
- carbon number of protons
- how to number carbons in a sugar ring
Information related to the topic How do you label carbons?
Here are the search results of the thread How do you label carbons? from Bing. You can read more if you want.
You have just come across an article on the topic How do you label carbons?. If you found this article useful, please share it. Thank you very much.