Are you looking for an answer to the topic “How is a negative ion formed from a neutral atom?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Atoms are neutral; they contain the same number of protons as electrons. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.13.1. Negative ion formation. By adding an electron to an atom or a molecule, some energy states below the vacuum level can become populated, i.e. negative ions. Although the atom is not stable, it has a net negative charge of −e.An ion is formed when an atom loses or gains electrons. This causes an otherwise neutral atom to acquire a charge: a positively charged cation is created if electrons are lost. a negatively charged anion is created if electrons are gained.

Table of Contents
How is a negative ion formed?
13.1. Negative ion formation. By adding an electron to an atom or a molecule, some energy states below the vacuum level can become populated, i.e. negative ions. Although the atom is not stable, it has a net negative charge of −e.
How does a neutral atom become an ion?
An ion is formed when an atom loses or gains electrons. This causes an otherwise neutral atom to acquire a charge: a positively charged cation is created if electrons are lost. a negatively charged anion is created if electrons are gained.
What’s an Ion?
Images related to the topicWhat’s an Ion?

How does a positive ion form from a neutral atom?
A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell.
What type of atoms form negative ions?
…
Forming negative ions.
| Atom name | Ion name |
|---|---|
| Fluorine | Fluoride |
| Bromine | Bromide |
| Iodine | Iodide |
| Sulfur | Sulfide |
What is most likely to form a negative ion?
In the given elements chlorine (2,8,7) is a non metal with highest electronegativity. Hence, it is most likely to form a negative ion with charge −1.
How are ions formed from atoms?
Ions are formed by the addition of electrons to, or the removal of electrons from, neutral atoms or molecules or other ions; by combination of ions with other particles; or by rupture of a covalent bond between two atoms in such a way that both of the electrons of the bond are left in association with one of the …
How does an atom become a positive or a negative ion quizlet?
-An atom becomes a positive ion by gaining an electron; it becomes a negative ion by losing an electron.
See some more details on the topic How is a negative ion formed from a neutral atom? here:
How does an atom become a negative ion? – Quora
Atom are electrically neutral .It is so because of presence of positive charge carries proton equal to negative charge called electron.
Ions are formed when neutral atom gains or lose … – Vedantu
Hint: Ions are of two types, cation and anion. The cation has a positive charge and the anion has a negative charge. These charges are due to the addition or …
Ionic Bonding | Chemistry I – Simple Book Production
As you have learned, ions are atoms or molecules bearing an electrical charge. A cation (a positive ion) forms when a neutral atom loses one or more …
9.4: Ionic Bonding – Chemistry LibreTexts
Ions form when atoms gain or lose electrons. Since electrons are negatively charged, an atom that loses one or more electrons will become …
What is the process of a neutral atom acquiring either a positive or negative charge?
A neutral atom or group of atoms becomes an ion by gaining or losing one or more electrons or protons. Since the electron and proton have equal but opposite unit charges, the charge of an ion is always expressed as a whole number of unit charges and is either positive or negative.
Atoms form ions (Chemistry) – Binogi
Images related to the topicAtoms form ions (Chemistry) – Binogi
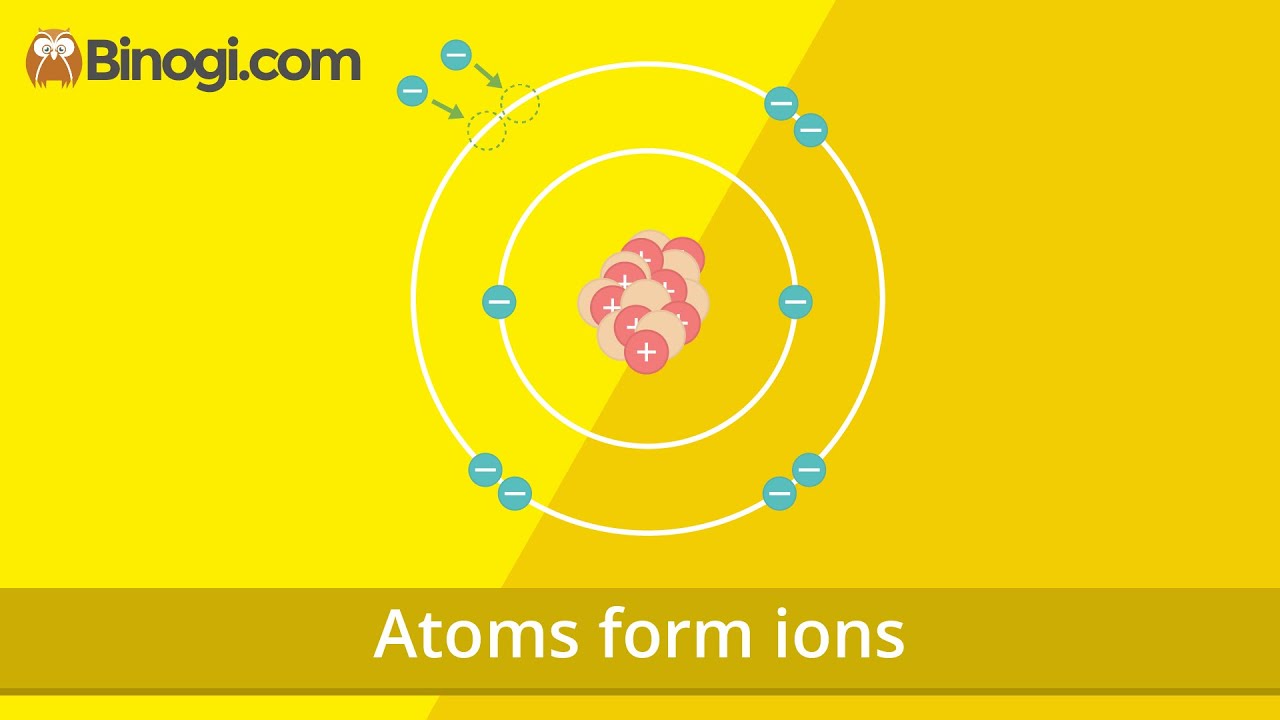
Why is a positive ion positive and how does a neutral atom become a positive ion?
Neutral atoms can be turned into positively charged ions by removing one or more electrons. A neutral sodium atom, for example, contains 11 protons and 11 electrons. By removing an electron from this atom we get a positively charged Na+ ion that has a net charge of +1.
Are Positive ions are formed from neutral atoms by loss of electrons?
Answer. Positive ions are formed from neutral atoms by loss of electrons.
What is formed when a neutral atom loses an electron?
Picture 3.1 A neutral atom that loses an electron becomes a positive ion. In ordinary matter, the number of electrons in an atom is the same as the number of protons. The positive and negative charges balance each other.
What tends become negative ions?
And thus nitrogen, oxygen, fluorine, chlorine, etc. TEND to form negative ions, i.e. N3− , O2− , F− , and Cl− ; clearly the PARENT atoms (or molecules) are oxidizing species.
What happens when a neutral atom gains an electron?
If a neutral atom gains electrons, then it will become negatively charged. If a neutral atom loses electrons, then it become positively charged. 6.
How do you know which ions are positive and negative?
To find the ionic charge of an element you’ll need to consult your Periodic Table. On the Periodic Table metals (found on the left of the table) will be positive. Non-metals (found on the right) will be negative.
What is a negatively charged ion called?
The atom that has lost an electron becomes a positively charged ion (called a cation), while the atom that picks up the extra electron becomes a negatively charged ion (called an anion).
GCSE Chemistry – Formation of Ions #13
Images related to the topicGCSE Chemistry – Formation of Ions #13
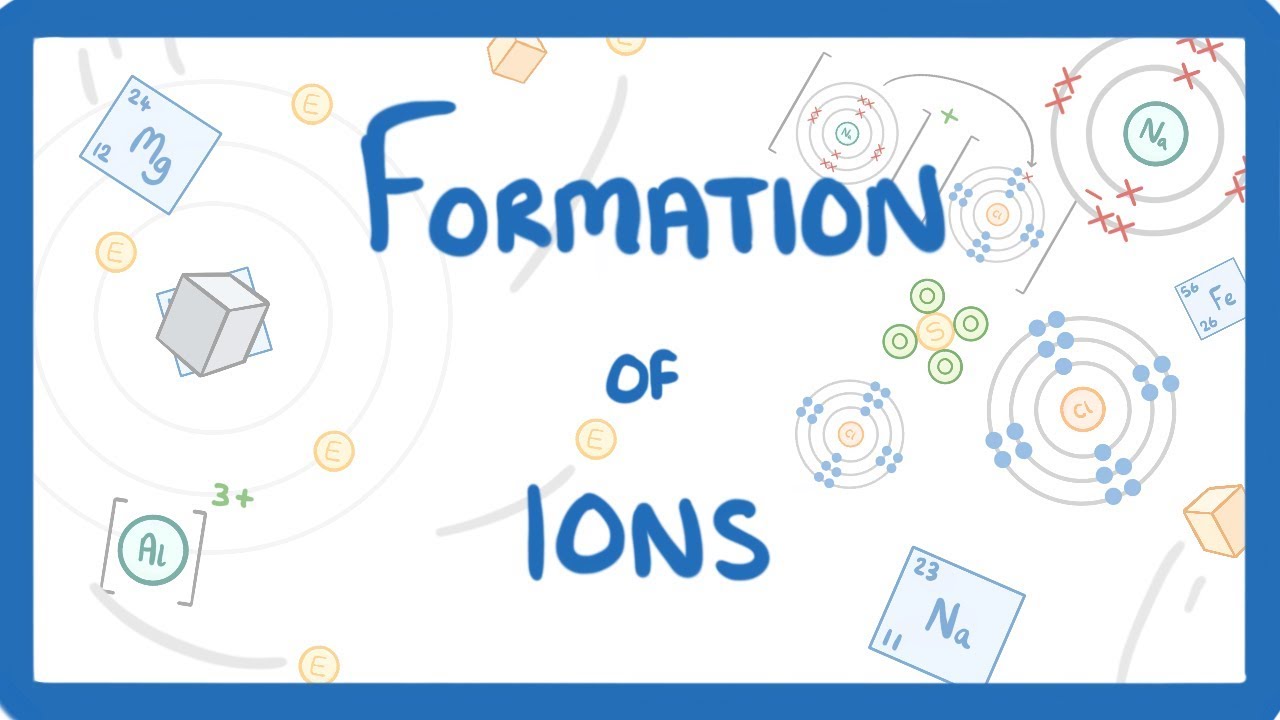
How are ions formed quizlet?
Ions are formed when atoms lose or gain electrons in order to fulfill the octet rule and have full outer valence electron shells.
How is an ionic bond formed?
ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
Related searches to How is a negative ion formed from a neutral atom?
- how is a negative ion formed from a neutral atom of oxygen
- how does an atom become an ion
- are all atoms neutral
- how is a negative ion formed from a neutral atom of potassium
- how is a negative ion formed
- how does an atom become a negative ion
- how is a negative ion formed from a neutral atom of lithium
- how is a negative ion formed from a neutral atom of chlorine
- how is the positive ion called
- how are ions formed
- how can a neutral atom become a cation
- how is a negative ion formed from a neutral atom of carbon
- how does an atom become a positive or a negative ion
- what is the difference between an atom and an ion
- how does an atom become a positive ion
Information related to the topic How is a negative ion formed from a neutral atom?
Here are the search results of the thread How is a negative ion formed from a neutral atom? from Bing. You can read more if you want.
You have just come across an article on the topic How is a negative ion formed from a neutral atom?. If you found this article useful, please share it. Thank you very much.