Are you looking for an answer to the topic “How is electron affinity measured?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
The electron affinity is calculated by adding the energy gap and the ionization energy. However, the energy gap determined by PAS is often smaller than the actual energy gap by 0.2–1 eV, and difference is interpreted as the exciton binding energy [11, 12]. Thus, the electron affinity is overestimated by PAS.The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. X ( g ) + e − → X − ( g ) + E . A . Therefore, the electron affinity of chlorine is – 349 KJ/mol.The electron and ion con- centrations are measured by the application of a magnetic field. The temperature is measured with an optical pyrometer. The apparent electron affinity is obtained by plotting Ln K vs. 1/T.
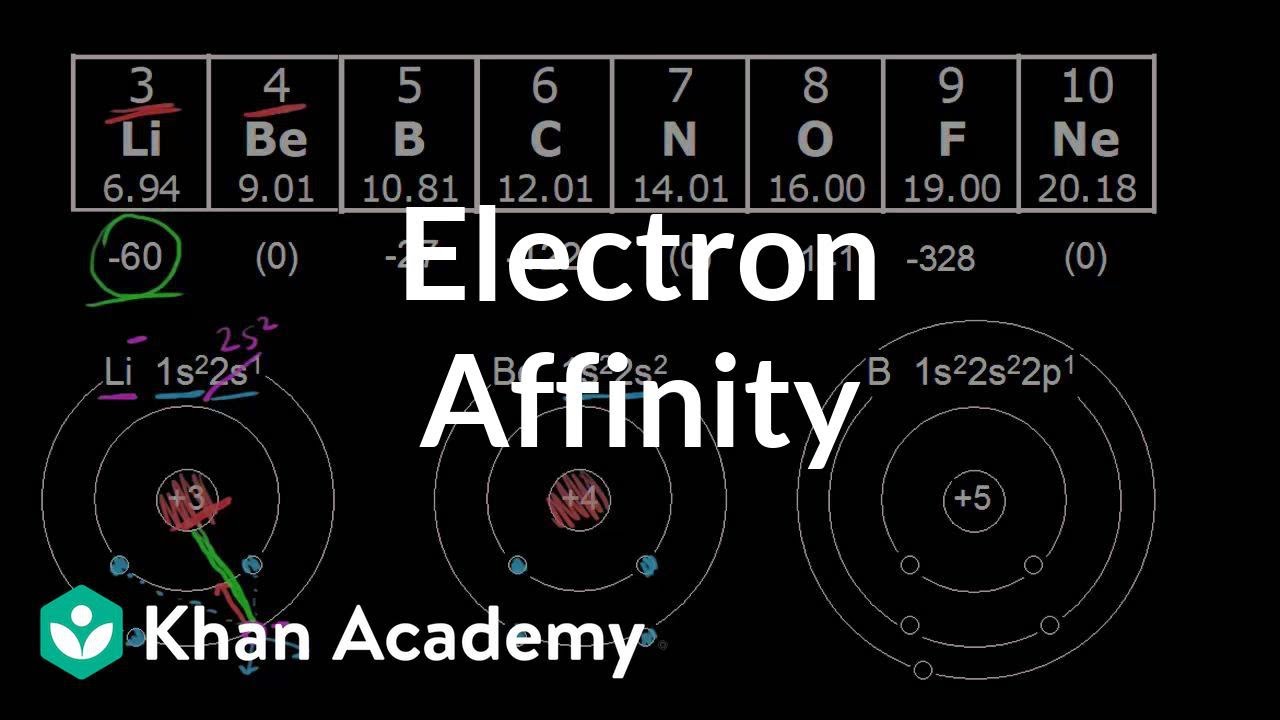
Table of Contents
How do you determine electron affinity?
The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. X ( g ) + e − → X − ( g ) + E . A . Therefore, the electron affinity of chlorine is – 349 KJ/mol.
How is electron affinity measured experimentally?
The electron and ion con- centrations are measured by the application of a magnetic field. The temperature is measured with an optical pyrometer. The apparent electron affinity is obtained by plotting Ln K vs. 1/T.
Electron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
Images related to the topicElectron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy

What unit is electron affinity measured in?
Electron affinity is the amount of energy required to detach one electron from a negatively charged ion of an atom or molecule. It is indicated using the symbol Ea and is usually expressed in units of kJ/mol.
How do you determine greatest electron affinity?
Patterns in Electron Affinity. Electron affinity increases upward for the groups and from left to right across periods of a periodic table because the electrons added to energy levels become closer to the nucleus, thus a stronger attraction between the nucleus and its electrons.
Can electronegativity be measured experimentally?
Electronegativity cannot be measured experimentally. However, various numerical scales to express electronegativity were developed by many scientists. The Pauling scale of electronegativity is the one used most widely.
Is electron affinity same as electronegativity?
Electronegativity is defined as a chemical property which decides the propensity of an atom to attract an electron. In the year 1932, Linus Pauling proposed the concept of electronegativity. Electron affinity is defined as the amount of energy liberated when a molecule or neutral atom acquires an electron from outside.
What are electrons measured in?
electron volt, unit of energy commonly used in atomic and nuclear physics, equal to the energy gained by an electron (a charged particle carrying unit electronic charge) when the electrical potential at the electron increases by one volt. The electron volt equals 1.602 × 10−12 erg, or 1.602 × 10−19 joule.
See some more details on the topic How is electron affinity measured? here:
Experimental Determination of the Electron Affinity of Fluorine
Both methods were used to determine the electron affinity of fluorine, E(F), the best measured value being E(F)=S2.1±2.1 kcal/mole. In the first method the …
Electron affinity – Wikipedia
The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an …
Electron Affinity | Introduction to Chemistry
Electron affinity is measured for atoms and molecules in the gaseous state only, since in the solid or liquid states their energy levels would be changed by …
electron affinity – Chemguide
The electron affinity is a measure of the attraction between the incoming electron and the nucleus – the stronger the attraction, the more energy is …
What is electron affinity in periodic table?
Key Points. The electron affinity of an atom or molecule is the propensity for that particle to gain an electron. This is an exothermic process for all non-noble gas elements. There are general trends in electron affinity across and down the periodic table of elements.
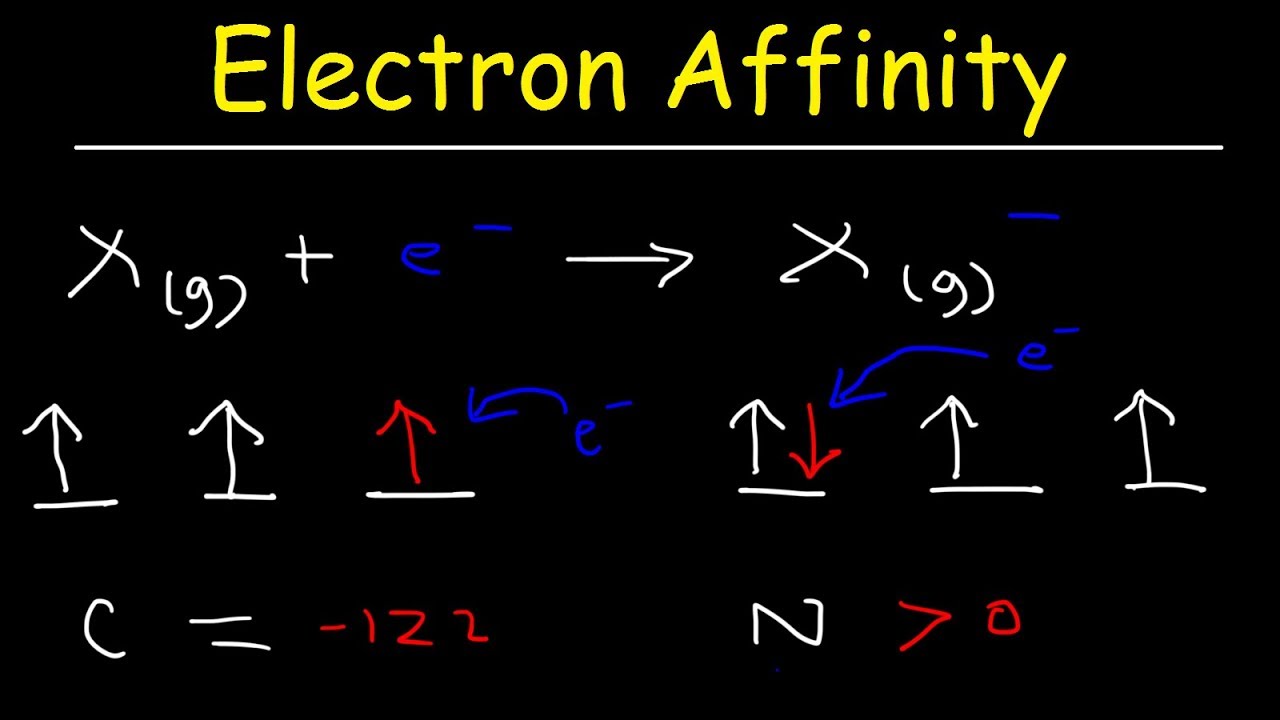
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry
Is electron affinity the same as ionization energy?
Chemical Bond Energy Considerations
Ionization energy: the energy required to remove an electron from a neutral atom. Electron affinity: the energy change when a neutral atom attracts an electron to become a negative ion.
Are electron affinity and ionization energy related?
The smaller the first electron affinity, the easier an atom gains electrons. The greater the electron affinity, the weaker is the ability of an atom to gain electrons. The ionization energy shows the ability of an atom to loose its electrons. This ability can explain the metal activity of an element.
What is the relationship between ionization energy electronegativity and electron affinity?
Elements with both high I.E. and E.A. are reluctant to lose their electrons, therefore they tend to gain them instead (highly electronegative). Where X = Electronegativity, I = Ionization Energy, E = Electron Affinity. This equation shows the positive correlation between all three trends. Hope this helps!
What has the lowest electron affinity?
The correct answer is Argon. Argon has all filled orbitals as well as a filled valence shell. As a result, it doesn’t want to lose or gain any electrons. Hence, argon has the lowest electron affinity.
Which has higher electron affinity F or Cl?
Electronegativity of fluorine is greater than that of chlorine but electron affinity of chlorine is greater than that of fluorine.
What is Pauling scale in chemistry?
Pauling scale is a numerical scale of electronegativities based on bond-energy calculations for different elements joined by covalent bonds. Electronegativity is the power of an atom when in a molecule to attract eletrons to itself. Electronegativity is a property of a chemical element, not of an electron.
What is electron affinity? | Chemistry | Extraclass.com
Images related to the topicWhat is electron affinity? | Chemistry | Extraclass.com

What are Pauling units?
The most commonly used method of calculation is that originally proposed by Linus Pauling. This gives a dimensionless quantity, commonly referred to as the Pauling scale (χr), on a relative scale running from 0.79 to 3.98 (hydrogen = 2.20).
How did Pauling measured electronegativity?
Pauling based his scale on thermochemical data, particularly bond energies, which allowed him to calculate differences in electronegativity between atoms in a covalent bond. He assigned a value of 4.0 to fluorine, the most electronegative element, and calculated other values with respect to that.
Related searches to How is electron affinity measured?
- positive electron affinity
- electron affinity of chlorine
- how to check electron affinity
- how to determine electron affinity
- electron affinity trend down a group
- how is electron affinity measured
- electron affinity periodic table
- how to explain electron affinity
- what does electron affinity measure
- electron affinity of n
- electron affinity of k
- electron affinity trend
- electron affinity of br
- what is electron affinity
Information related to the topic How is electron affinity measured?
Here are the search results of the thread How is electron affinity measured? from Bing. You can read more if you want.
You have just come across an article on the topic How is electron affinity measured?. If you found this article useful, please share it. Thank you very much.