Are you looking for an answer to the topic “How is MgCl2 an ionic compound?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
MgCl2 is an Ionic compound, not a covalent. Since the gap in electronegativity value between magnesium (1.31) and chlorine (3.16) is large, therefore, the bond formed in Magnesium chloride is Ionic. It consists, of two ions, Mg2+ and 2Cl–, both of these are held together by ionic bonding.Magnesium chloride. An inorganic compound consisting of one magnesium and two chloride ions.MgCl2 is an ionic compound because chemical bonds in the molecule are formed by the transfer of electrons among Mg and Cl atoms. Magnesium is an alkaline earth metal that loses two electrons to form magnesium cation while chlorine being a non-metal accepts the one-electron each to form a chloride ion.

Table of Contents
Why is MgCl2 an ionic compound?
MgCl2 is an Ionic compound, not a covalent. Since the gap in electronegativity value between magnesium (1.31) and chlorine (3.16) is large, therefore, the bond formed in Magnesium chloride is Ionic. It consists, of two ions, Mg2+ and 2Cl–, both of these are held together by ionic bonding.
What type of compound is MgCl2?
Magnesium chloride. An inorganic compound consisting of one magnesium and two chloride ions.
Ionic Bond in MgCl2 English
Images related to the topicIonic Bond in MgCl2 English

Does MgCl2 contain ionic or covalent bonds?
MgCl2 is an ionic compound because chemical bonds in the molecule are formed by the transfer of electrons among Mg and Cl atoms. Magnesium is an alkaline earth metal that loses two electrons to form magnesium cation while chlorine being a non-metal accepts the one-electron each to form a chloride ion.
What is the chemical bonding of MgCl2?
Ionic bonding in MgCl2 magnesium chloride.
Is magnesium and chlorine ionic?
The oppositely charges of the magnesium and chloride ions attract each other and form ionic bonds.
How is MgCl2 formed?
Hence MgCl2 is formed by the transfer of electrons from electron rich to electron deficient species. Magnesium chloride only conducts electricity when in molten or liquid state. It is an ionic compound and in liquid state it releases its ions when enables them to conduct electricity.
How does an ionic bond form?
ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
See some more details on the topic How is MgCl2 an ionic compound? here:
Why is MgCl2 an ionic compound? – Quora
MgCl2 is an ionic compound because this compound is formed by transfer of electrons between magnesium atom and chlorine atoms.
Is MgCl2 Ionic or Covalent? – What’s Insight
MgCl2 is an ionic compound. The magnesium atom loses two electrons to produce the Mg2+ ion and each chlorine accepts one electron to form the Cl– ion.
Is MgCl2 Ionic or Covalent? – Techiescientist
MgCl2 is an ionic compound because chemical bonds in the molecule are formed by the transfer of electrons among Mg and Cl atoms. Magnesium is an alkaline earth …
Is MgCl2 ionic or covalent? | Study.com
MgCl2 is an ionic compound. Magnesium is a metal with a positive charge of +2. Remember, metals are found on the left side of the periodic table.
Why is MgCl2 called magnesium chloride?
MgCl2 can be extracted from sea water or brine and is chemically named as Magnesium Chloride. Magnesium Chloride is also called Magnesium dichloride or magnesium (II) chloride or Chloromagnesite and has one magnesium (Mg) and two chloride ions (Cl-).
Is MgCl2 (Magnesium chloride) Ionic or Covalent?
Images related to the topicIs MgCl2 (Magnesium chloride) Ionic or Covalent?
How will you describe and identify ionic and covalent compound?
Ionic compounds are (usually) formed when a metal reacts with a nonmetal (or a polyatomic ion). Covalent compounds are formed when two nonmetals react with each other. Since hydrogen is a nonmetal, binary compounds containing hydrogen are also usually covalent compounds.
Is NaCl ionic and covalent?
Ionic bonds usually occur between metal and nonmetal ions. For example, sodium (Na), a metal, and chloride (Cl), a nonmetal, form an ionic bond to make NaCl. In a covalent bond, the atoms bond by sharing electrons. Covalent bonds usually occur between nonmetals.
What is ionic compound properties?
Ionic compounds form when atoms connect to one another by ionic bonds. An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. One atom in the bond has a partial positive charge, while the other atom has a partial negative charge.
Which of the following describe an ionic compound?
Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids they are almost always electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized.
Is magnesium covalent or ionic?
…
Is MgO ionic or covalent?
| Name of Molecule | Magnesium Oxide |
|---|---|
| Chemical formula | MgO |
| Bond type | Ionic |
| Molar mass | 40.304 g/mol |
| Nature | Basic |
Will magnesium form covalent or ionic compounds?
Yes Magnesium does form covalent bonds.
How is MgCl2 formed by transfer of electrons?
Magnesium chloride a ionic compound is formed by the transferring of electrons. magnesium having 2 valence electron and chlorine having 1 valence electron are interchanged with each other so the resultant compound is the MgCl2.
Draw the Lewis Structure of MgCl2 (magnesium chloride)
Images related to the topicDraw the Lewis Structure of MgCl2 (magnesium chloride)
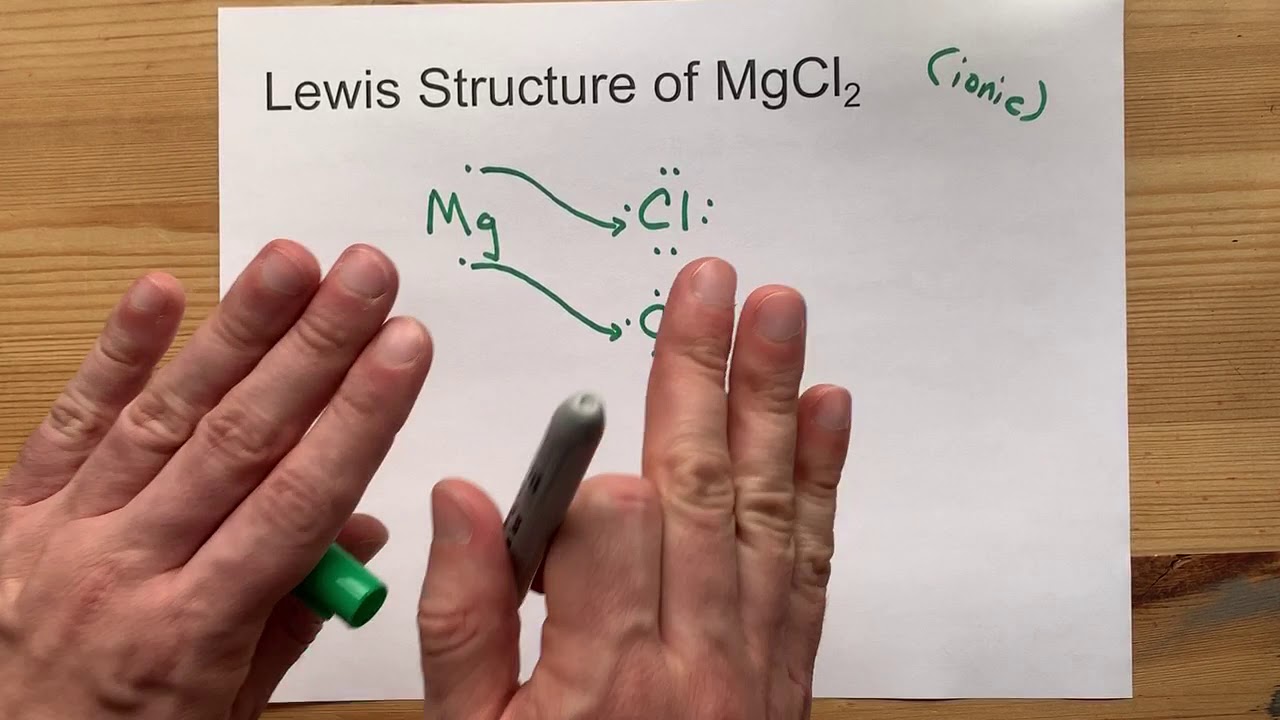
How is MgCl2 formed by transfer of electrons Why does a solution of MgCl2 conduct electricity?
Magnesium Chloride is formed by the transfer of electrons. They form an ionic bond. An ionic bond is a type of bond in which there is complete transfer of electrons from one atom to to other atom(s). The solution of Magnesium Chloride conducts electricity because it is electrolytic in nature.
Is magnesium oxide ionic or covalent?
No, magnesium oxide (MgO) is not considered to be bonded via covalent bonds. Moreover, the chemical bond between magnesium and oxygen in magnesium oxide is ionic in nature. This is a consequence of the large difference in the electronegativities of magnesium and oxygen.
Related searches to How is MgCl2 an ionic compound?
- how is mgcl2 an ionic compound
- mgcl2 ionic compound name
- is h2o ionic or covalent
- mgcl2 lewis structure
- mgcl2 type of bond
- co ionic or covalent
- is mgcl2 polar or nonpolar
- is mgcl2 an ionic bond
- does mgcl2 contain an ionic bond
- mgcl2 second elementatom metal or non metal
- magnesium chloride formula
- is na2s ionic or covalent
- is mgcl2 an ionic or covalent compound
- nh3 ionic or covalent
Information related to the topic How is MgCl2 an ionic compound?
Here are the search results of the thread How is MgCl2 an ionic compound? from Bing. You can read more if you want.
You have just come across an article on the topic How is MgCl2 an ionic compound?. If you found this article useful, please share it. Thank you very much.