Are you looking for an answer to the topic “How is NaCl an ionic compound?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
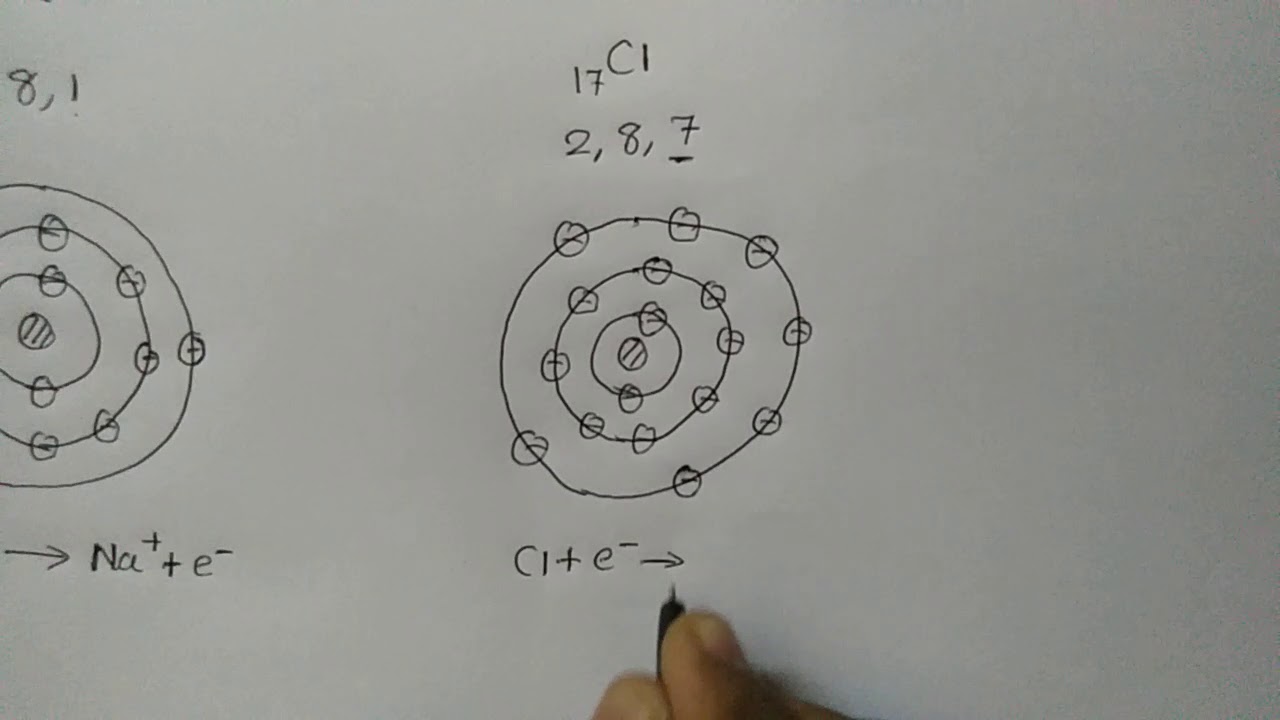
Sodium chloride (NaCl) is a typical ionic compound. The picture below shows both a sodium and a chlorine ion. Sodium has 1 electron in its outermost shell, and chlorine has 7 electrons. It is easiest for sodium to lose its electron and form a +1 ion, and for chlorine to gain an electron, forming a -1 ion.Sodium chloride (NaCl) is an ionic compound. Because of the complete transfer of electrons taking place from a metal, sodium (Na), to a nonmetal, chlorine (Cl), the (Na+ and Cl— ions) are held together by the electrostatic force of attraction that caused the formation of ionic bond in NaCl.Sodium is a metal that is present in group 1 (alkali metals) and chlorine is a nonmetal that is present in group 17 (halogens). The number of outermost electrons is one in sodium and seven in chlorine. NaCl is an ionic compound and so the formation takes place due to transfer of electrons from sodium to chlorine atom.

Table of Contents
Why is NaCl an ionic compound?
Sodium chloride (NaCl) is an ionic compound. Because of the complete transfer of electrons taking place from a metal, sodium (Na), to a nonmetal, chlorine (Cl), the (Na+ and Cl— ions) are held together by the electrostatic force of attraction that caused the formation of ionic bond in NaCl.
How can you say that NaCl is an ionic compound?
Sodium is a metal that is present in group 1 (alkali metals) and chlorine is a nonmetal that is present in group 17 (halogens). The number of outermost electrons is one in sodium and seven in chlorine. NaCl is an ionic compound and so the formation takes place due to transfer of electrons from sodium to chlorine atom.
Formation of an Ionic Compound
Images related to the topicFormation of an Ionic Compound

How is NaCl a ionic bond?
Ionic Bond in Sodium Chloride
An electron is transferred from sodium to chlorine. Sodium becomes a positive ion and chlorine becomes a negative ion. Positive and negative ions attract each other and form an ionic bond and the compound sodium chloride.
Is NaCl molecular or ionic?
Sodium Chloride (NaCl) is a pure ionic compound and not a covalent compound. The two atoms transfer their electrons to form ions, Na+ and Cl-.
What type of bond is NaCl compound?
Many atoms and groups of atoms in chemical compounds are ions that have an electrical charge because of their unequal numbers of protons and electrons. Cations are positively charged ions and anions are negatively charged ions.
How NaCl is formed explain?
Sodium chloride is a compound formed from the ionic bonding of sodium and chloride. Sodium chloride is formed when sodium atoms interact with chlorine atoms. Sodium will donate an electron (which is a negatively-charged particle) to chlorine. This makes sodium slightly positive and chlorine slightly negative.
Is NaCl ionic or polar covalent?
Sodium Chloride (NaCl) which is an ionic compound acts as a polar molecule. Usually, the large difference in electronegativities in sodium and chlorine makes their bond polar. Besides, in sodium chloride, Na has a +1 charge and Cl has a -1 charge and the bond is a strong one.
See some more details on the topic How is NaCl an ionic compound? here:
4.3: Sodium Chloride and Ionic Bonds – Chemistry LibreTexts
Most common ionic compounds such as sodium chloride are hard solids because the ions of which they are composed are relatively small and packed …
Covalent Compounds – University of Hawaii at Manoa
Ionic compounds, such as sodium chloride (NaCl), are formed by a transfer of electrons that creates ions. Ions exert electrostatic force on each other, which …
NaCl is an ionic compound. How is an ionic bond is formed in …
No. of electrons in valance shell of Na=1. No. of electrons in valance shell of Cl=7. So, Sodium donates its valance electron to chlorine and (both completing …
Ionic Bond – an overview | ScienceDirect Topics
Sodium chloride is an example of an ionic solid. A sodium atom, which has 11 protons and 11 electrons, has a single valence electron in its 3s subshell. A …
Ionic Bond in Sodium Chloride NaCl Std 9 10
Images related to the topicIonic Bond in Sodium Chloride NaCl Std 9 10
How are ionic compounds formed?
An ionic compound is a compound that is formed by ionic bonding. Ionic bonding occurs through a process called electron transfer, where one atom gives electrons to another.
Why is NaCl a stable compound?
Sodium chloride is a solid food additive that is more stable than either of its constituent parts, all because the outermost energy levels of its atoms are filled with electrons.
Is NaCl covalent compound?
NaCl (Sodium Chloride) is formed due to ionic bonding, when the atoms Na and Cl transfer their electrons giving rise to ions such as Na+ and Cl–. Hence, single-single electrons are transferred here. NaCl is not a covalent bond.
What is ionic compound properties?
Ionic compounds form when atoms connect to one another by ionic bonds. An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. One atom in the bond has a partial positive charge, while the other atom has a partial negative charge.
Is this compound ionic or covalent?
Compounds containing two elements (so called binary compounds) can either have ionic or covalent bonding. If a compound is made from a metal and a non-metal, its bonding will be ionic. If a compound is made from two non-metals, its bonding will be covalent.
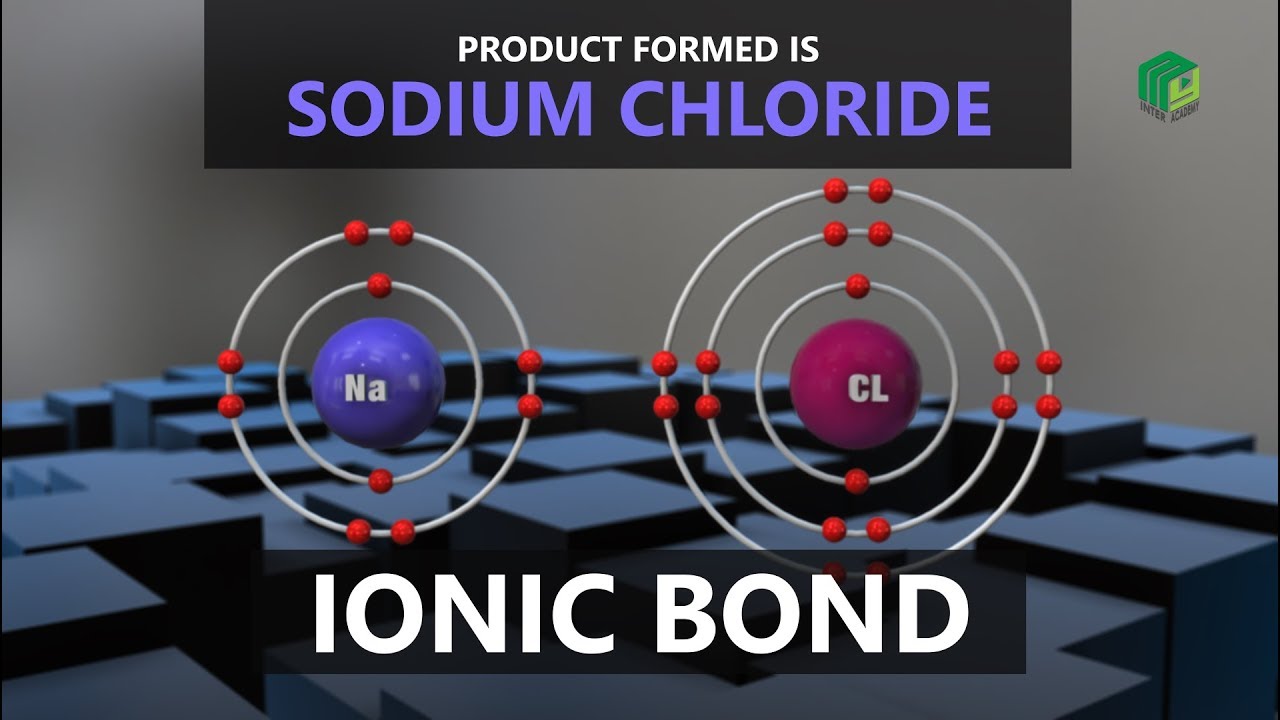
| Ionic Bond | My Inter Academy |
Images related to the topic| Ionic Bond | My Inter Academy |
Is NaCl an ionic salt?
Sodium chloride, also known as table salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. It is commonly used as a condiment and food preservative. Salt can be created by adding two very reactive elements together: sodium (Na(s) metal and chlorine (Cl2(g) gas.
How do ions form ionic bonds?
Ionic bonds are formed through the exchange of valence electrons between atoms, typically a metal and a nonmetal. The loss or gain of valence electrons allows ions to obey the octet rule and become more stable. Ionic compounds are typically neutral. Therefore, ions combine in ways that neutralize their charges.
Related searches to How is NaCl an ionic compound?
- ionic compounds
- is h2o ionic or covalent
- what type of bond is sodium chloride
- how is nacl an ionic bond
- ionic bonds and compounds
- how is nacl an ionic compound
- why is nacl an ionic bond
- nacl metal or nonmetal
- bonding in ionic compounds
- co2 ionic or covalent
- does nacl contain an ionic bond
- how do you know that nacl is an ionic compound
- is sucrose ionic or covalent
- how is salt an ionic compound
- formation of ionic compounds
Information related to the topic How is NaCl an ionic compound?
Here are the search results of the thread How is NaCl an ionic compound? from Bing. You can read more if you want.
You have just come across an article on the topic How is NaCl an ionic compound?. If you found this article useful, please share it. Thank you very much.