Are you looking for an answer to the topic “How much energy does it take to melt 2 kg of ice refer to table of constants for water?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Keep Reading

Table of Contents
How much energy does it take to melt 2 kg of ice answers?
Therefore, to melt 2 kg of ice 835.48 KJ of heat is required.
What is the heat required to melt 2 kg of ice?
Therefore, to melt 2kg of ice 835. 48kJ of heat is required.
Calculating the Energy to Melt Ice
Images related to the topicCalculating the Energy to Melt Ice

How much energy does it take to melt 1kg of ice?
How many Joules of energy do you need to melt all the ice into a pure liquid along the path from B to C on the graph? Answer: For 1 kilogram of ice ,which equals 1000 grams, we need 333 Joules/gram x 1000 grams = 333,000 Joules.
How much energy does it take for ice to melt?
A total of 334 J of energy are required to melt 1 g of ice at 0°C, which is called the latent heat of melting. At 0°C, liquid water has 334 J g−1 more energy than ice at the same temperature. This energy is released when the liquid water subsequently freezes, and it is called the latent heat of fusion.
What quantity of heat is needed to convert 2 kg of ice at to steam at 100c?
Now, we know that the total heat required to convert 2 kg ice from -10 degree Celsius to 100 degree Celsius is the sum of heat required by the individual process. Therefore, the total energy required is 6061.2 Joules.
What is latent heat of fusion apex?
Answer: The latent Heat of Fusion is the change inenthalpy resulting from heating a given quantity of a substance to change its state from a solid to a liquid. The temperature at which this occurs is the melting point.
How much energy does it take to change ice to water?
Ice is the solid phase of water (H2O). Under normal conditions, the melting point at the Earth’s surface is 0°C. To change the phase of H2O, a certain amount of energy must be absorbed or released. In order to melt ice of 0°C to water of 0°C, a high amount of energy is needed, equivalent to 334 Joules for each gram.
See some more details on the topic How much energy does it take to melt 2 kg of ice refer to table of constants for water? here:
How much energy does it take to melt 2 kg of ice? – Answers
It takes about 80 calories to melt a gram of ice, so melting 2 kg of ice would take about 160 kilocalories. If ice is place on wood how long …
How much energy does it take to melt 2 kilograms of ice?
We know that latent heat required for melting of ice is 80Cg-1. Two Kg would be 2000 g and the heat energy needed would be 2000g X 80 = 160000C.
How much energy does it take to boil 100 ml of water Refer to …
… 2 kg of ice to 2 …
What is water heat capacity?
The exact value of the specific heat capacity of water is 4182 J/kg°C. Now, water is quite commonly occurring and an important substance in our life. Therefore, there is a special way to identify the total amount of heat energy needed to raise one gram of water by a calorie (one degree Celsius).
What is the thermal capacity of 2kg of water in J C?
The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree Celsius (°C). The specific heat capacity of water is 4,200 joules per kilogram per degree Celsius (J/kg°C).
How much heat does it take to melt 1.25 kg of ice?
With this in mind, plug in the values, remembering that m is mass (in g) so you need to convert 1.25 kg to g, and kJ/mol must be converted to kJ/g using the molar mass of water ( 18.02gmol ). Now, type this in your calculator, and you will get your answer in kJ. With significant figures, you will get: 419 kJ .
What is the amount of thermal energy needed to melt 1kg of a substance called?
The amount of heat energy required to change the state of 1 kg of a substance at its melting point is called the specific latent heat of the substance. The specific latent heat of water is: l f = 3 ⋅ 34 × 10 5 J k g − 1 for fusion (solid→liquid) or freezing (liquid→solid)
Heating Curve and Cooling Curve of Water – Enthalpy of Fusion Vaporization
Images related to the topicHeating Curve and Cooling Curve of Water – Enthalpy of Fusion Vaporization
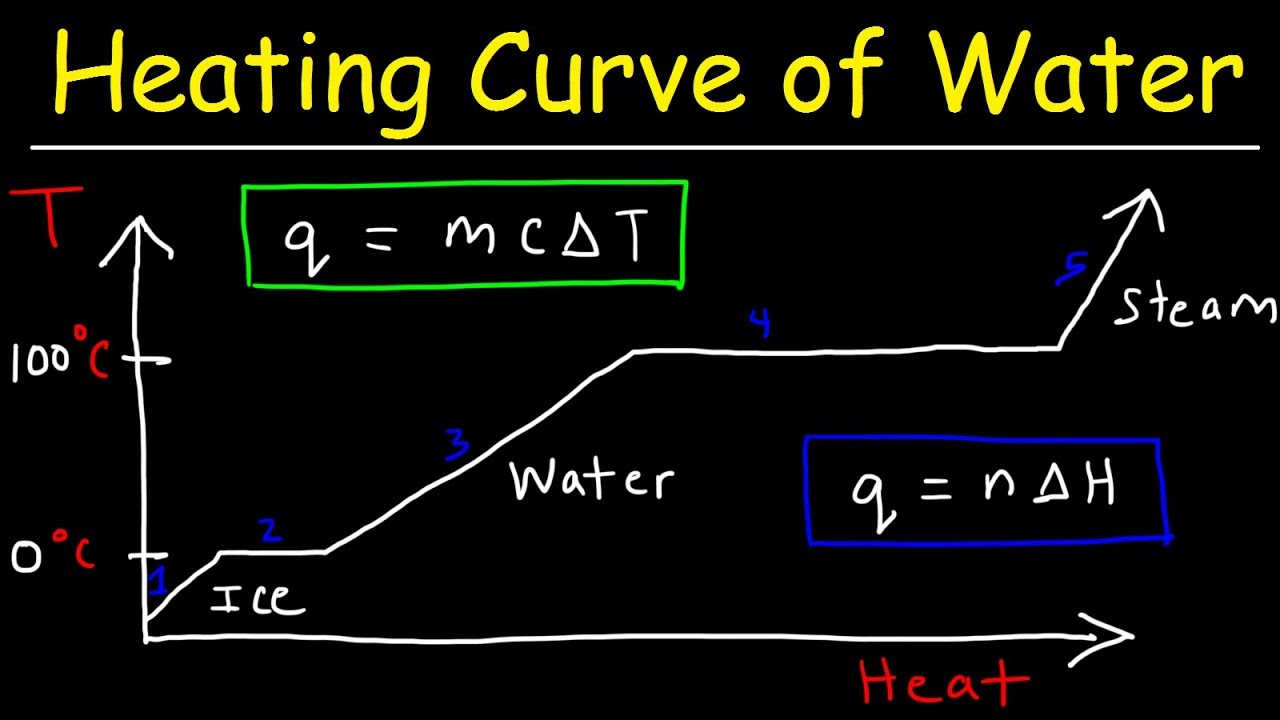
How much energy does it take to melt 0.5 kg of ice?
(2)✎ 167000 joules of energy are required to melt 0.5kg of ice.
How much energy does it take to melt 1g of ice?
– To melt 1 gram of ice requires 80 calories. (A calorie is defined as the amount of energy needed to raise one gram of water 1°C.) – The change from liquid to ice is called solidification.
How much energy takes to convert 1g solid water to 1g liquid water this is known as the heat of fusion?
Heat of Fusion. For water at its normal freezing point of 0 ºC, the specific heat of Fusion is 334 J g–1. This means that to convert 1 g of ice at 0 ºC to 1 g of water at 0 ºC, 334 J of heat must be absorbed by the water.
What is the specific heat of ice?
| Substance | Specific Heat (cal/gram C) | Specific Heat (J/kg C) |
|---|---|---|
| Ice (0 C) | 0.50 | 2093 |
| sandy clay | 0.33 | 1381 |
| dry air (sea level) | 0.24 | 1005 |
| quartz sand | 0.19 | 795 |
What is the specific heat of steam?
The specific heat capacity, or the amount of heat needed to raise the temperature of a specific substance in a specific form one degree Celsius, for water is 4.187 kJ/kgK, for ice 2.108 kJ/kgK, and for water vapor (steam) 1.996 kJ/kgK.
What is the relation between specific heat capacity C and heat capacity C of a body of mass m?
The heat capacity and the specific heat are related by C=cm or c=C/m. The mass m, specific heat c, change in temperature ΔT, and heat added (or subtracted) Q are related by the equation: Q=mcΔT. Values of specific heat are dependent on the properties and phase of a given substance.
What does the latent heat of vaporization represent Brainly?
Answer. The latent heat of vapouriztion of a liquid is the quantity of heat in joules required to convert 1kg of the liquid (at its boiling point) to vapour or gas, without any change in temperature at atmospheric pressure. HOPE THIS ANSWER WOULD BE HELPFUL TO U!!!
How much energy does it take to melt 1.5 kg of lead that is at its melting point?
The enthalpy of fusion (energy need to change state from solid to liquid) of lead is 4.77 kJ/mol (so it takes 4.77 kJ to melt one mole of lead). We need to find the number of moles ( n ) of lead in 1.5kg ( m ).
What is thermal energy Answers apex?
Thermal energy is a measure of total kinetic energy. Thermal energy is a flow of heat.
How do you calculate melting time of ice?
If I wish to determine how much time ‘t’ it will take to melt two kilos of ice on a stow at 1000 W, i will have to use the following expression: t = L’m / p t = 335000 J/kg ‘ 2 kg / 1000 W t = 670 seconds = 11.2 minutes.
Calculate the Specific Latent Heat of Fusion of Ice
Images related to the topicCalculate the Specific Latent Heat of Fusion of Ice

What is energy formula?
Energy is defined as the capacity to do work. Formula. The energy stored in an object due to its position and height is known as potential energy and is given by the formula: P.E. = mgh. Unit.
How much energy would it take to melt 75g of ice?
The amount of heat required to melt 75 g of ice is 26586 J.
Related searches to How much energy does it take to melt 2 kg of ice refer to table of constants for water?
- which of the following would be a good situation in which to apply hess’s law
- which of the following would be a good situation in which to apply hesss law
- what information does the latent heat of vaporization give apex
- how much heat energy is required to heat 2 kg of water at 20 c to 100 c
- how is stoichiometry used to calculate energy absorbed when a mass of liquid boils
- how much energy is required to heat 2 kg of water through the following changes
- what best describes the enthalpy of formation of a substance
- which statement describes the potential energy diagram of an endothermic reaction
- what does the latent heat of fusion represent
Information related to the topic How much energy does it take to melt 2 kg of ice refer to table of constants for water?
Here are the search results of the thread How much energy does it take to melt 2 kg of ice refer to table of constants for water? from Bing. You can read more if you want.
You have just come across an article on the topic How much energy does it take to melt 2 kg of ice refer to table of constants for water?. If you found this article useful, please share it. Thank you very much.