Are you looking for an answer to the topic “How much heat does it take to melt a gram of ice?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
A total of 334 J of energy are required to melt 1 g of ice at 0°C, which is called the
.– The change from solid to liquid is called fusion, or melting. – To melt 1 gram of ice requires 80 calories. (A calorie is defined as the amount of energy needed to raise one gram of water 1°C.) – The change from liquid to ice is called solidification.Using the equation for a change in temperature and the value for water from Table 1, we find that Q = mLf = (1.0 kg)(334 kJ/kg) = 334 kJ is the energy to melt a kilogram of ice.

How much heat does it take to melt 1g of ice?
– The change from solid to liquid is called fusion, or melting. – To melt 1 gram of ice requires 80 calories. (A calorie is defined as the amount of energy needed to raise one gram of water 1°C.) – The change from liquid to ice is called solidification.
How much heat would it take to melt a 1 kg block of ice?
Using the equation for a change in temperature and the value for water from Table 1, we find that Q = mLf = (1.0 kg)(334 kJ/kg) = 334 kJ is the energy to melt a kilogram of ice.
Final Temperature of Ice and Water Mixture – How Many Grams of Ice Will Melt?
Images related to the topicFinal Temperature of Ice and Water Mixture – How Many Grams of Ice Will Melt?

How much heat does it take to melt 100g of ice?
The specific heat of melting of ice is 334 J/g, so melting 100g of ice will take 33,400 J. The specific heat of vaporization of water is 2230 J/g, so evaporating 100g of water will take 223,000 J.
What is the minimum amount of heat needed to melt 10 grams of ice?
So,to convert 10g of ice at 0∘C to same amount of water at the same temperature, heat energy required would be 80⋅10=800 calories.
Is more energy required to melt one gram?
Answer. Therefore, it is required more energy to boil one gram of water at 100 degrees than to melt one gram of ice at 0 degrees.
How much energy does it take to raise the temperature of 1 gram of ice by 1 C?
Since the specific heat of ice is 0.50 cal/g-oC, that means that 0.50 calories is needed to raise 1g of ice 1oC.
What is the heat of fusion of ice?
(1) 333.55 J/g (heat of fusion of ice) = 333.55 kJ/kg = 333.55 kJ for 1 kg of ice to melt, plus.
See some more details on the topic How much heat does it take to melt a gram of ice? here:
How Much Heat Energy Is Needed To Melt 1 Gram Of Ice?
– To melt 1 gram of ice requires 80 calories. (A calorie is defined as the amount of energy needed to raise one gram of water 1°C.) – The change …
How do you calculate the heat required to melt ice?
Answer Expert Verified Enthalpy of fusion of ice is 334 Joule per gram. So, 8.35 kJ of …
Heat of Fusion Example Problem – Melting Ice – ThoughtCo
Answer: The amount of heat required to melt 25 grams of ice is 8,350 Joules or 2,000 calories. Note: Heat of fusion should be a positive value.
Heat Energy Required to Turn Ice into Steam by Ron Kurtus
How much heat would be required to melt the 50g of ice? The latent heat for melting ice is 80 cal/g. That means that 1g of ice requires 80 cal of heat to melt.
What is the specific heat of ice?
| Substance | Specific Heat (cal/gram C) | Specific Heat (J/kg C) |
|---|---|---|
| Ice (0 C) | 0.50 | 2093 |
| sandy clay | 0.33 | 1381 |
| dry air (sea level) | 0.24 | 1005 |
| quartz sand | 0.19 | 795 |
At what temperature does ice melt?
At temperatures above 32°F (0°C), pure water ice melts and changes state from a solid to a liquid (water); 32°F (0°C) is the melting point. For most substances, the melting and freezing points are about the same temperature.
How much heat is needed to melt 500g ice?
How much heat, Q, is required to melt 500 g of ice at 0 °C ? By definition Q=mL where the heat of fusion of ice is Lice = 3.33 x 10° J/kg Therefore, Q=(0.5 kg). (3.33 x 10°) = 166.5 kJ Page 2 … ::: .
How much heat is required to change 1g of ice at exactly 0 C to steam at 100 C?
So, the correct answer is 716 Cal.
How much heat is required to melt 100 grams of ice at its melting point if the heat of fusion is 80 cal g?
…
The Formula for the Heat of Fusion:
| \Delta H_f | heat of fusion |
|---|---|
| m | mass |
How Much Thermal Energy Is Required To Heat Ice Into Steam – Heating Curve Chemistry Problems
Images related to the topicHow Much Thermal Energy Is Required To Heat Ice Into Steam – Heating Curve Chemistry Problems

How do you calculate the amount of heat needed to melt?
- Heat of fusion is the amount of energy in the form of heat needed to change the state of matter from a solid to a liquid (melting.)
- The formula to calculate heat of fusion is: q = m·ΔHf
How do you calculate melting time of ice?
If I wish to determine how much time ‘t’ it will take to melt two kilos of ice on a stow at 1000 W, i will have to use the following expression: t = L’m / p t = 335000 J/kg ‘ 2 kg / 1000 W t = 670 seconds = 11.2 minutes.
How do I calculate specific heat?
The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1 °C. The formula is Cv = Q / (ΔT ⨉ m) .
Will it take more energy to evaporate one gram of water or melt one gram of ice?
1 Answer. You’d need about 7 times more energy to vaporize 1 g of water at 100∘C than to melt the same amount of ice at 0∘C . Mathematically, you would know this from the difference between water’s latent heat of vaporization and latent heat of fusion.
How can water vapor become ice?
Once water vapor is in the atmosphere, low temperatures cause the vapor to either condense into a liquid or undergo deposition to form ice crystals. Water droplets and ice crystals in the atmosphere form clouds, which are moved around the planet by air currents.
What is the amount of energy in Joules need to vaporize 1 gram of a liquid called?
energy known as the latent heat of vaporization is required to break the hydrogen bonds. At 100 °C, 540 calories per gram of water are needed to convert one gram of liquid water to one gram of water vapour under normal pressure.
How many calories are needed to melt 1g of ice at 0 C?
– The change from solid to liquid is called fusion, or melting. – To melt 1 gram of ice requires 80 calories.
What is the amount of heat released by 1.00 gram of liquid water?
This means that to convert 1 g of ice at 0 ºC to 1 g of water at 0 ºC, 334 J of heat must be absorbed by the water. Conversely, when 1 g of water at 0 ºC freezes to give 1 g of ice at 0 ºC, 334 J of heat will be released to the surroundings.
How much energy is required to raise the temperature of the melted ice to θ C?
The specific heat capacity of water is 4,200 joules per kilogram per degree Celsius (J/kg°C). This means that it takes 4,200 J to raise the temperature of 1 kg of water by 1°C.
How much energy does it take to melt 1kg of ice?
How many Joules of energy do you need to melt all the ice into a pure liquid along the path from B to C on the graph? Answer: For 1 kilogram of ice ,which equals 1000 grams, we need 333 Joules/gram x 1000 grams = 333,000 Joules.
how much ice is needed to cool water? Calculation
Images related to the topichow much ice is needed to cool water? Calculation
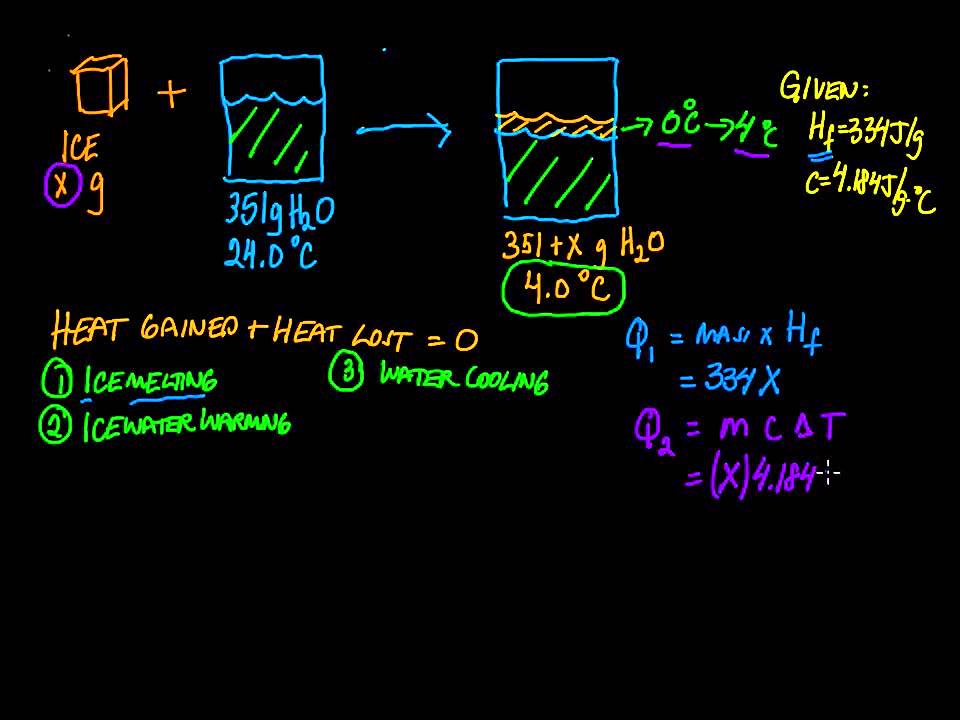
How much energy does it take to melt ice?
In order to melt ice of 0°C to water of 0°C, a high amount of energy is needed, equivalent to 334 Joules for each gram. The same amount of energy is released in the atmosphere or ground when water freezes to ice.
How do you calculate latent heat of ice?
Latent heat calculation
The specific latent heat is different for solid to liquid transition and liquid to gas transition. For example, if we want to turn 20 g of ice into water we need Q = 20 g * 334 kJ/kg = 6680 J of energy.
Related searches to How much heat does it take to melt a gram of ice?
- how much heat is required to melt 250g of ice the latent heat of fusion is 80 calg
- how much heat does it take to melt a gram of ice cream
- how to calculate heat of fusion of ice
- heat required to melt ice calculator
- heat of fusion of ice j/kg
- specific heat of ice
- molar heat of fusion of ice
- how much heat does it take to melt a gram of ice weigh
- how much heat does it take to melt a gram of ice cost
- how much heat is needed to melt ice at 0c if the sample weighs 134g
- heat of fusion of ice jkg
- how much heat does it take to melt a gram of ice look like
- how much heat is needed to melt 1 gram of ice in joules
Information related to the topic How much heat does it take to melt a gram of ice?
Here are the search results of the thread How much heat does it take to melt a gram of ice? from Bing. You can read more if you want.
You have just come across an article on the topic How much heat does it take to melt a gram of ice?. If you found this article useful, please share it. Thank you very much.