Are you looking for an answer to the topic “How much heat is released during neutralization?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Enthalpy of neutralization is the heat evolved when one gram equivalent of the acid is completely neutralized by a base in dilute solution. The chemical reaction is given below. 13.7 kcal of heat is liberated out and is the heat of neutralization for all strong acids and bases.Neutralization reactions are generally exothermic and thus ΔH is negative. Heat measurements are performed by carrying out the reaction in a special container called a calorimeter. The heat (Q) given off by the neutralization reaction is absorbed by the reaction solution and the calorimeter.SInce strong acids and strong bases are completely dissociated in solution, no formal bonds are being broken. The formation of two very strong covalent bonds between hydrogen and the hydroxide ion is responsible for the neutralization reaction’s exothermic character.

Table of Contents
Is heat released during neutralisation?
Neutralization reactions are generally exothermic and thus ΔH is negative. Heat measurements are performed by carrying out the reaction in a special container called a calorimeter. The heat (Q) given off by the neutralization reaction is absorbed by the reaction solution and the calorimeter.
Why is heat released in a neutralization reaction?
SInce strong acids and strong bases are completely dissociated in solution, no formal bonds are being broken. The formation of two very strong covalent bonds between hydrogen and the hydroxide ion is responsible for the neutralization reaction’s exothermic character.
Enthalpy Stoichiometry Part 2: How to Find Heat Released
Images related to the topicEnthalpy Stoichiometry Part 2: How to Find Heat Released

What is the heat of neutralization of HCl and NaOH?
The heat of neutralization of HCl by NaOH is -55.9 kJ/mol.
What is meant by heat of neutralization?
Definition of heat of neutralization
: the heat of reaction resulting from the neutralization of an acid or base especially : the quantity produced when a gram equivalent of a base or acid is neutralized with a gram equivalent of an acid or base in dilute solution.
Is neutralisation exothermic?
Some examples of exothermic reactions are: combustion (burning) neutralisation reactions between acids and alkalis.
How is the heat released in a neutralization reaction related to acid strength?
Enthalpy changes of neutralization are always negative – heat is released when an acid and and alkali react. For reactions involving strong acids and alkalis, the values are always very closely similar, with values between -57 and -58 kJ mol–1.
In which case heat of neutralisation is greater than 13.7 kcal?
a weak acid, with NaOH (aq.) is more than 13.7 kcal, in an exothermic reaction.
See some more details on the topic How much heat is released during neutralization? here:
Enthalpy Change of Neutralization – Chemistry LibreTexts
Enthalpy changes of neutralization are always negative – heat is released when an acid and and alkali react. For reactions involving strong …
How to Calculate the Molar Heat of Neutralization – Sciencing
The molar heat of neutralization is the amount of heat each mole of base added to the acid (or vice versa) causes the reaction to give off. (A …
Enthalpy of neutralization – Wikipedia
When a reaction is carried out under standard conditions at the temperature of 298 K (25 degrees Celsius) and 1 atm of pressure and one mole of water is formed, …
Enthalpy of Neutralisation Chemistry Tutorial – AUS-e-TUTE
Neutralisation reactions release heat, they are exothermic reactions, so the sign of the molar enthalpy of neutralisation is negative.
What is the molar heat of neutralization?
⚛ Molar enthalpy of neutralisation (molar heat of neutralization) is the energy liberated per mole of water formed during a neutralisation reaction.
What do neutralisation reactions produce?
An acid and alkali will neutralise each other and produce a salt and water. This is called a neutralisation reaction. The name of the salt produced can be worked out from the names of the acid and the alkali.
Using Calorimetry to Calculate Enthalpies of Reaction – Chemistry Tutorial
Images related to the topicUsing Calorimetry to Calculate Enthalpies of Reaction – Chemistry Tutorial
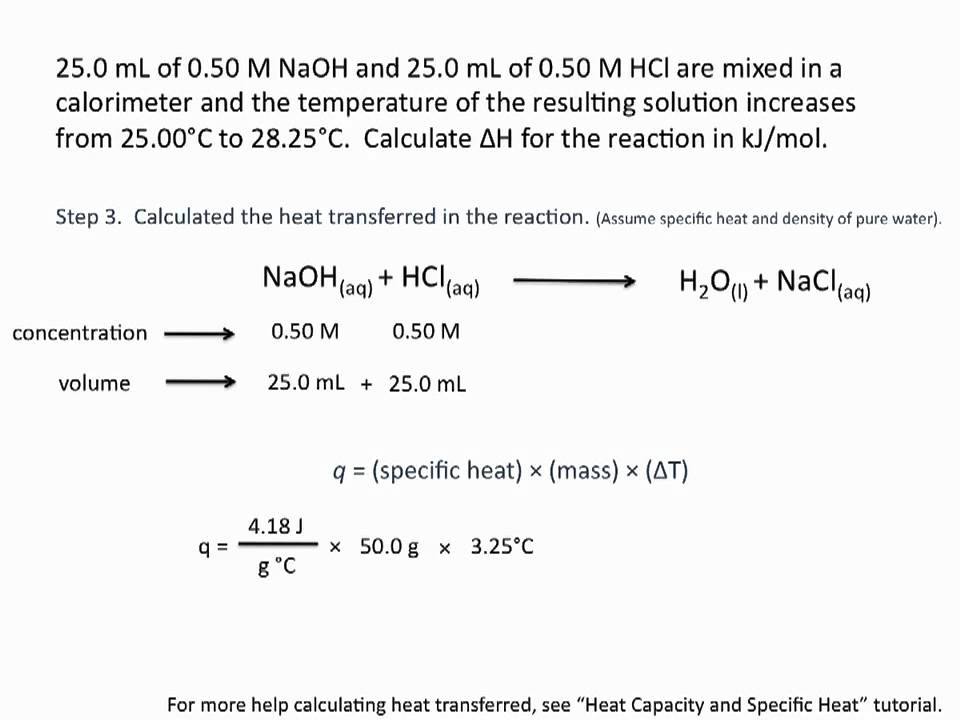
How do you calculate the heat of neutralization of h2so4 and NaOH?
…
Heat of neutralisation.
| ○ | 1 mole of HCl + 1 mole of NaOH | Heat released = 57.0kJ |
|---|---|---|
| ○ | 2 mole of HCl + 2 mole of NaOH | Heat released = 2 x 57.0kJ = 114kJ |
What is the enthalpy of neutralization of HCl and Koh?
and KOH. is 13.7 kCal. .
What is the value of heat of neutralization of NH4OH and HCl?
The enthalpy of neutralisation of NH4OH with HCl is -51.46 k.
Is the heat of neutralization the same?
For weak acids or bases, the heat of neutralization is pH-dependent. In the absence of any added mineral acid or alkali some heat is required for complete dissociation. The total heat evolved during neutralization will be smaller. The heat of ionization for this reaction is equal to (–12 + 57.3) = 45.3 kJ/mol at 25 °C.
What is the enthalpy of neutralization of a strong acid?
The enthalpy of neutralization of any strong acid and strong base is always constant, i.e. 57.1 kJ because both the acid and base undergo complete ionization.
When calculating the heat of neutralization the reaction mixture is?
Calculate the heat of neutralization using the fomula Q = mcΔT, where “Q” is the heat of neutralization, “m” is the mass of your acid, “c” is the specific heat capacity for aqueous solutions, 4.1814 Joules(grams x °C), and “ΔT” is the change in temperature you measured using your calorimeter.
Which neutralisation reactions are endothermic?
Acid base neutralization involves the formation of a salt and water. Such a process is inevitably exothermic. Bond-breaking is endothermic, whereas bond formation is exothermic.
Heat of Neutralization – Part 1 | Thermochemistry
Images related to the topicHeat of Neutralization – Part 1 | Thermochemistry

Do exothermic reactions get hotter?
An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.”
Does exothermic release energy?
Chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds in the reactants. Exothermic reactions are accompanied by an increase in temperature of the reaction mixture.
Related searches to How much heat is released during neutralization?
- heat of neutralization
- heat of neutralization of strong acid and strong base
- how much heat is released during neutralization reaction
- enthalpy of neutralization of hcl and naoh lab report
- how to calculate enthalpy of neutralization
- heat of neutralization calculator
- heat of neutralization lab report
- heat of neutralization of hcl and naoh
- why is heat released in a neutralization reaction
- enthalpy of neutralization table
- how much heat is released formula
- do neutralization reactions release heat
- how much heat is released by the neutralization reaction
- does neutralization produce heat
Information related to the topic How much heat is released during neutralization?
Here are the search results of the thread How much heat is released during neutralization? from Bing. You can read more if you want.
You have just come across an article on the topic How much heat is released during neutralization?. If you found this article useful, please share it. Thank you very much.