Are you looking for an answer to the topic “How much heat is required to change 1g of ice at exactly 0 C to steam at 100 C?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
So, the correct answer is 716 Cal.`1 gm` of ice at `0^@C` is converted to steam at `100^@C` the amount of heat required will be `(L_(“steam”) = 536 cal//g)`.CHANGES OF STATE OF WATER. – The change from solid to liquid is called fusion, or melting. – To melt 1 gram of ice requires 80 calories. (A calorie is defined as the amount of energy needed to raise one gram of water 1°C.)
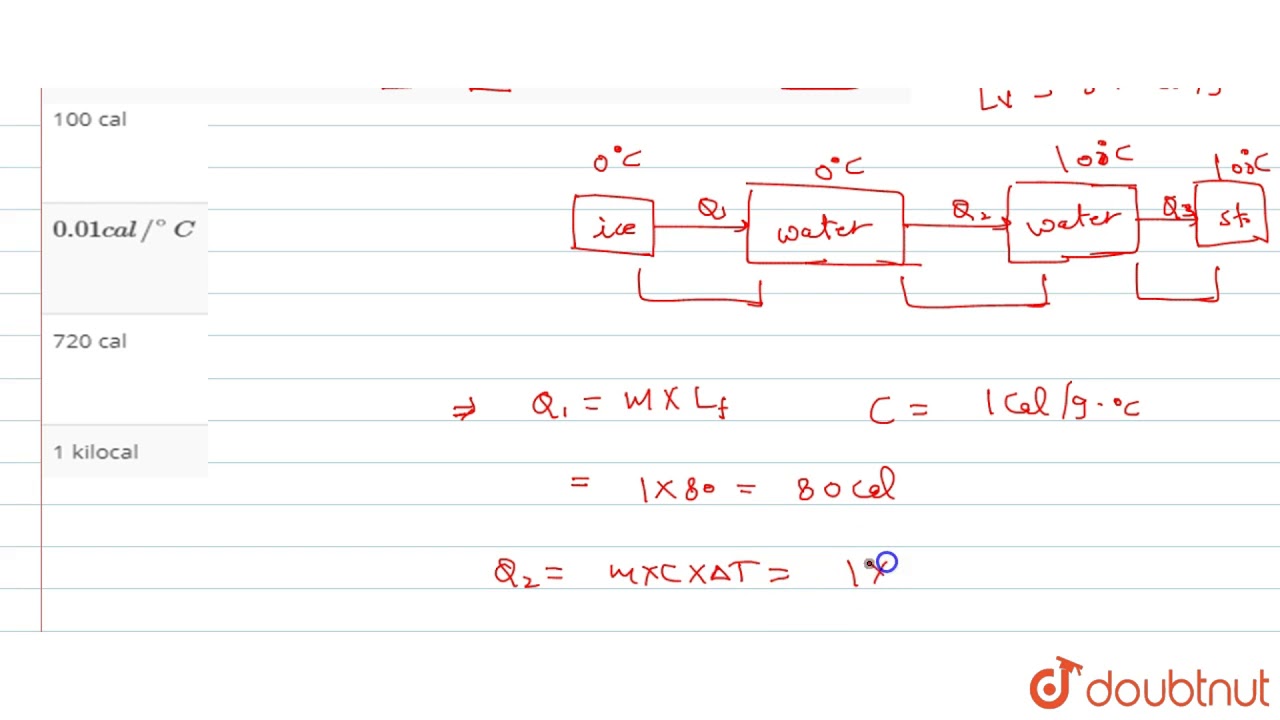
Table of Contents
What is the amount of energy required for 1gm of ice at 0 to become steam at 100?
`1 gm` of ice at `0^@C` is converted to steam at `100^@C` the amount of heat required will be `(L_(“steam”) = 536 cal//g)`.
How many calories of heat are required to change 1 gram of ice at 0 C to liquid water at 0 C?
CHANGES OF STATE OF WATER. – The change from solid to liquid is called fusion, or melting. – To melt 1 gram of ice requires 80 calories. (A calorie is defined as the amount of energy needed to raise one gram of water 1°C.)
Heat required to convert 1 g of ice at `0^(@)C` into steam at `100 ^(@)C` is
Images related to the topicHeat required to convert 1 g of ice at `0^(@)C` into steam at `100 ^(@)C` is

How many calories of heat is required to convert 1gm of ice at to water at?
Heat required in converting 1g of ice at −10∘C into steam at 100∘C is: Latent heat of fusion = 80 cal/g. Latent heat of vaporization = 540 cal/g.
What amount of energy is required to convert 500g of ice at 0 C to steam at 100 C?
g of ice at 0.00 °C to water vapour at 100.00 °C requires 301 kJ of energy.
How many calories are needed to melt 1g of ice at 0 C?
– The change from solid to liquid is called fusion, or melting. – To melt 1 gram of ice requires 80 calories.
When 1kg of ice at 0c is mixed with?
1 kg of ice at 0∘C is mixed with 1 kg of steam at 100∘C. What will be the composition of the system when thermal equilibrium is reached? Latent heat of fusion of ice = 3.36 × 105 J kg−1 and latent heat of vaporization of water = 2.26 × 106 J kg−1.
How much heat energy will be released when 1 gram of water at 0 C changes to 1 gram of ice at 0 C?
This means that to convert 1 g of ice at 0 ºC to 1 g of water at 0 ºC, 334 J of heat must be absorbed by the water. Conversely, when 1 g of water at 0 ºC freezes to give 1 g of ice at 0 ºC, 334 J of heat will be released to the surroundings.
See some more details on the topic How much heat is required to change 1g of ice at exactly 0 C to steam at 100 C? here:
heat required to convert 1g of ice at 0 degree celcius into …
Total heat required to convert 1 g of ice at 0°C into steam at 100°C is 716 cal. Explanation: Given Data: Mass of ice=1 g.
Heat Energy Required to Turn Ice into Steam by Ron Kurtus
How much heat would be required to raise 50g of ice to its melting point? … The ice temperature must be raised 10 degrees to reach 0oC. … Since the specific …
Total heat required to convert 1 g of ice at 0∘C into steam at …
Total heat required to convert 1 g of ice at 0∘C into steam at 100∘C is (Lsteam=536 cal/g) : (A) 100 cal (B) 0.01 kilo calorie (C) 716 cal (D) 1 kilo calorie.
Calculate Energy Required to Turn Ice Into Steam – ThoughtCo
c = (2.09 J/g·°C; ΔT = 0 °C – -10 °C (Remember, when you subtract a negative number, it is the same as adding a positive number …
How much energy does it take to raise the temperature of 1 gram of ice by 1 C?
Since the specific heat of ice is 0.50 cal/g-oC, that means that 0.50 calories is needed to raise 1g of ice 1oC.
How many joules of heat are needed to raise the temperature of the 0 C water to 1 C?
If we have one gram of water and one gram of ethanol both at 0ºC, it would take 4.18 joules of heat to raise the temperature of water to 1ºC, and only 2.18 joules for ethanol.
What is the amount of energy required for 1gm of ice?
The heat energy required to change the state of matter from solid to liquid is called Latent heat of Fusion. It is 80 Cal for 1 gram of ice.
What is the energy required for 1 gram of ice to become steam?
`1 gm` of ice at `0^@C` is converted to steam at `100^@C` the amount of heat required will be `(L_(“steam”) = 536 cal//g)`.
How much energy is absorbed when 10g of ice at0 0 C becomes steam at 100 0c?
question. Answer: Total heat is required to change 10 g ice at 0°C to steam at 100°C is 7200 cal.
THERMAL PROPERTIES Heat required in converting 1g of ice at 10°C into steam at 100°
Images related to the topicTHERMAL PROPERTIES Heat required in converting 1g of ice at 10°C into steam at 100°

How much energy is required to melt 10.0 g of ice at 0.0 C warm it to 100.0 C and completely vaporize the sample?
So,to convert 10g of ice at 0∘C to same amount of water at the same temperature, heat energy required would be 80⋅10=800 calories. So,to convert water at 100∘C to steam at 100∘C heat energy required will be 537⋅10=5370 calories.
How much energy is required to change a 40 g ice cube from ice at − 10oc to steam at 110oc?
How much thermal energy is required to change a 40 g ice cube from a solid at -10 oC to steam at 110 oC? ΔQ = 0.04 kg*(0.49 kcal/(kgoC))*10 oC = 0.196 kcal.
What quantity of heat is needed to convert 2 kg of ice at to steam at 100c?
Now, we know that the total heat required to convert 2 kg ice from -10 degree Celsius to 100 degree Celsius is the sum of heat required by the individual process. Therefore, the total energy required is 6061.2 Joules.
How many calories are needed to change the temperature of 1g of water 1c?
So if sw=1 cal/g∘C , then you would need 1 cal to raise the temperature of 1 g of water by 1∘C .
How many calories are required at 0 degrees C to melt an ice cube with a mass of 25g?
Answer: The amount of heat required to melt 25 grams of ice is 8,350 Joules or 2,000 calories.
How many calories are needed to vaporize 1g of boiling water at 100 C?
However, 540 calories of energy are required to convert that 1 g of water at 100˚ C to 1 g of water vapor at 100˚ C. This is called the latent heat of vaporization.
When 1 kg of ice at 0 degree Celsius melts to water at zero degree Celsius what is the resulting change in entropy?
=293cal/k.
Which contains more heat 1 kg of ice at 0oC or 1 kg of water at 0oC give reason?
1 g of water at 0oC has more heat than 1 g of ice at 0oC. This is because ice at 0oC absorbs 360 J of heat energy to convert into water at 0oC.
What is water heat capacity?
The exact value of the specific heat capacity of water is 4182 J/kg°C. Now, water is quite commonly occurring and an important substance in our life. Therefore, there is a special way to identify the total amount of heat energy needed to raise one gram of water by a calorie (one degree Celsius).
How much energy does it take to convert 1g liquid water to 1g water vapor?
ocean temperatures
… energy known as the latent heat of vaporization is required to break the hydrogen bonds. At 100 °C, 540 calories per gram of water are needed to convert one gram of liquid water to one gram of water vapour under normal pressure. Water can evaporate at temperatures below the boiling…
How much heat is required to change 10 kg ice at -10 °C to steam at 100 °C ?
Images related to the topicHow much heat is required to change 10 kg ice at -10 °C to steam at 100 °C ?

How many J of heat does it take to boil 1 g of water completely to steam?
For water at its boiling point of 100 ºC, the heat of vaporization is 2260 J g-1. This means that to convert 1 g of water at 100 ºC to 1 g of steam at 100 ºC, 2260 J of heat must be absorbed by the water.
How do you calculate latent heat of ice?
Find the latent heat of fusion, Lf, according to Lf = q ÷ m by dividing the heat, q, absorbed by the ice, as determined in step 3, by the mass of ice, m, determined in step 4. In this case, Lf = q / m = 2293 J ÷ 7.0 g = 328 J/g.
Related searches to How much heat is required to change 1g of ice at exactly 0 C to steam at 100 C?
- how many calories are required to change one gram of 0
- how much heat is required to convert ice to steam
- calculate the energy required to convert 1 70 g of ice originally at
- latent heat of ice
- calculate the amount of heat needed to convert 230 0 g of ice at to water at 0c
- the thermal capacity of 40 g of aluminium specific heat 0 2 calgc is
- how much heat is required to change 10g of ice
- heat required to convert water to steam
- how much heat is needed to convert 1 kg of ice into steam
- how much heat in kilocalories is needed to convert
- how much heat is released when 60 0 g of steam at 235 c is converted to water at 100 c
- how much heat is released when 60.0 g of steam at 235 c is converted to water at 100 c
Information related to the topic How much heat is required to change 1g of ice at exactly 0 C to steam at 100 C?
Here are the search results of the thread How much heat is required to change 1g of ice at exactly 0 C to steam at 100 C? from Bing. You can read more if you want.
You have just come across an article on the topic How much heat is required to change 1g of ice at exactly 0 C to steam at 100 C?. If you found this article useful, please share it. Thank you very much.