Are you looking for an answer to the topic “Is Br more reactive than Cl in Sn2?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Keep Reading

Table of Contents
Does Br or Cl react faster?
Although the bromine nucleus is more positively charged than the chlorine nucleus, the increase in the radius and the extra shielding in the bromine atom outweigh this factor, which means that an electron is more easily attracted into the outer shell of a chlorine atom than that of a bromine atom, so chlorine is more …
Which is more reactive in SN2 reaction?
CH3−Br is more reactive towards SN2 mechanism. CH3−Br is methyl halide. The order of reactivity towards SN2 mechanism is methyl halide > primary alkyl halide > secondary alkyl halide > tertiary alkyl halide.
Sn2 Stereochemistry
Images related to the topicSn2 Stereochemistry

What effect does the nature of the leaving group Cl or Br have on SN2 reactivity of SN1 reactivity?
Explanation: Nucleophilic substitution reactions whether it is SN1 or SN2 favour better leaving group. With a better leaving group, the substitution occurs faster. This also applies to elimination reactions E1 and E2.
Which halide reacts fastest in an SN2 reaction?
The Reaction Rate Of The SN2 Reaction Is Fastest For Small Alkyl Halides (Methyl > Primary > Secondary >> Tertiary) Finally, note how changes in the substitution pattern of the alkyl halide results in dramatic changes in the rate of the reaction.
Is Br or Cl A better nucleophile?
Reviving this question! #468 in 1001 in Orgo Chem Examkrackers says that Br- is a better nucleophile than Cl-, but #458 says that Br- is a better leaving group than Cl-. I get that Br- is bigger than Cl- and can therefore better stabilize the negative charge, making it a better leaving group.
Which is better leaving group Br or Cl?
Bulky groups are better leaving groups as when they leave, the steric factor gets stabilized. Br- is pretty bulky making it a better leaving group.
What is the order of reactivity of alkyl halides in SN2 reaction?
So,order of reactivity of alkyl halide in Sn2 reaction is RI>RBr>RCl>RF.
See some more details on the topic Is Br more reactive than Cl in Sn2? here:
Chapter 8 Notes: Nucleophilic Substitution and Elimination – Pdx
larger leaving groups react faster. I- > Br- > Cl- >> F-; poor leaving groups (unstable anions, strong bases) OH- , RO- , NH2-. SN2 Mechanism – Nucleophiles.
Alkyl Halide Reactivity – MSU chemistry
X change from Cl to Br to I (F is relatively unreactive) … The most reactive nucleophiles are said to be more nucleophilic than less reactive members of …
Sn2
Among the halogens, the leaving group reactivity sequence is F < Cl < Br < I for reactions performed in protic solvents. The tendency of fluoride ion to act ...
The Effect of Halogen Atoms on the Reactivity of Other …
to be more reactive than potassium dibromo- acetate, showingthat -bromine atoms decrease. Sn2 reactivity more than «-chlorine atoms in this.
Which of the following is least reactive towards SN2 mechanism?
Aryl halides are least reactive towards nucleophilic substitution due to resonance stabilisation.
Which of the following is the correct order of reactivity towards SN2 reaction?
Hence, the correct order of decreasing SN2 reactivity is: RCH2X>R2CHX>R3CX.
Is Br a good leaving group for SN2?
4) Leaving group: Br -, a very good leaving group. Decision: The data suggests Sn2, except for the solvent. Methanol is a polar protic solvent, which is good for a Sn1 reaction.
Which alkyl halide reacts slowest in an SN2 reaction?
OCH2CH3 versus better Nuc! Unimolecular: Dealing with the rate of the reaction. The rate of any SN2 reaction is directly linked to the concentration only one species, the alkyl halide (RX) undergoing substitution. This is the only species involved in the rate-determining (thus slowest) step.
Organic chemistry – Ranking nucleophilicity and Sn2 reactivity
Images related to the topicOrganic chemistry – Ranking nucleophilicity and Sn2 reactivity

Is Br a good leaving group?
Good leaving groups are weak bases. They’re happy and stable on their own. Some examples of weak bases: halide ions (I-, Br-, Cl-) water (OH2), and sulfonates such as p-toluenesulfonate (OTs) and methanesulfonate (OMs).
What is the order of reactivity of halides?
Hence, the reactivity order is RI>RBr>RCl.
Is Br an electrophile or nucleophile?
Bromine as an electrophile
Since two identical bromine atoms are joined together in the bromine molecule there is no reason why one atom should pull the bonding pair of electrons towards itself – they must be equally electronegative and so there won’t be any separation of charge, + or -.
Is bromine bigger than chlorine?
Bromine is located below chlorine in group 17, which means that a bromine atom is larger than a chlorine atom. This of course implies that the outermost electrons are located further away from the nucleus in bromine’s case.
Why is I better leaving group than Cl?
Iodine is a better leaving group than other halogen atoms due to its larger size. Due to larger size, charge density decreases and it becomes stable. So, its a better leaving group.
Is bromine or chlorine a weaker base?
Bases are electron donors. The strength of the base is ranked on the basis of its ability to donate electron pairs. Chlorine (Cl) is a stronger base than bromide(Br).
Which is the best leaving group in a substitution reaction of an alkyl halide Cl Br if?
Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. Iodide, which is the least basic of the four common halides (F, Cl, Br, and I), is the best leaving group among them.
Why is benzyl chloride reactive in SN1 and SN2?
Benzyl chloride is highly reactive towards the SN1 reaction because the intermediate benzyl carbocation formed in the slowest step is stabilized through resonance.
What is the order of reactivity of alkyl halides ie for Cl F Br I for SN1 reaction?
Why is it that the order of reactivity of alkyl halide towards SN1 reactions is tertiary? Tertiary alkyl halide > secondary alkyl halide > primary alkyl halide. This order is basically based on the stability of carbocations which are formed after the removal of halide ion.
SN2 Reaction Mechanisms
Images related to the topicSN2 Reaction Mechanisms
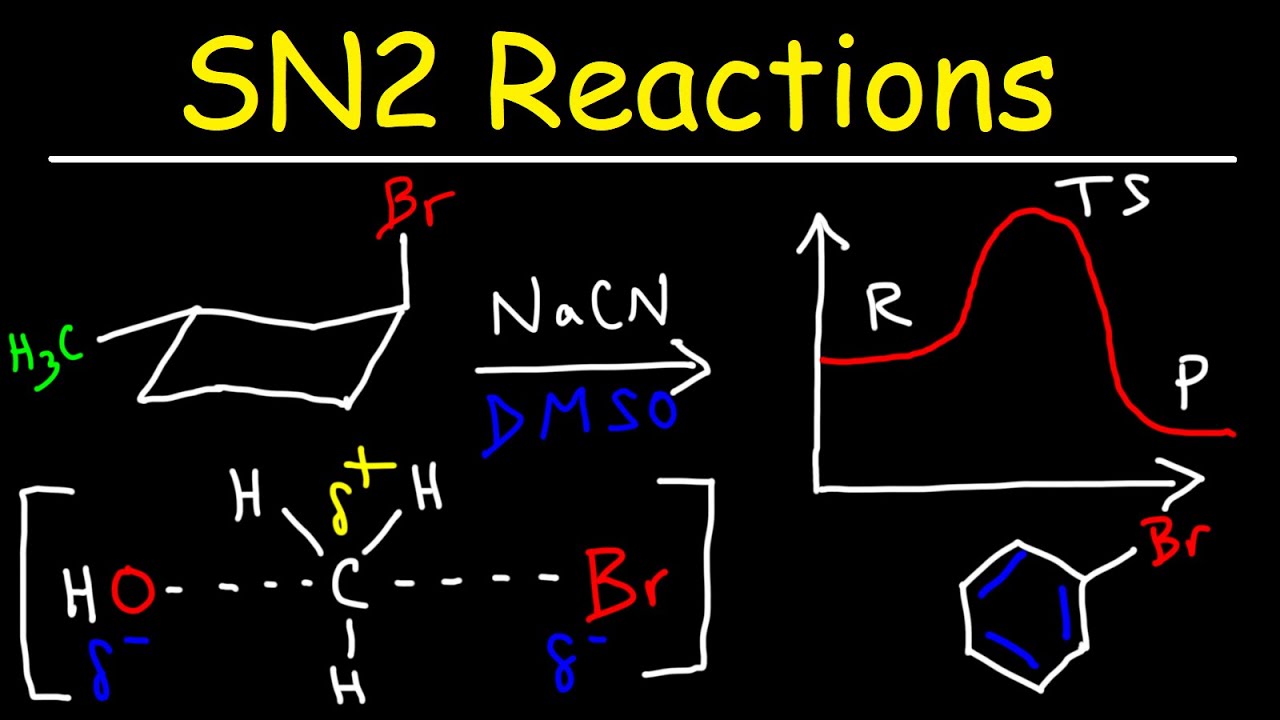
What is the order of increasing reactivity of alkyl halide?
We would find a common substitution product, C2H5–CN, in all cases, but the speed or rate of the reaction would increase in the order: Cl < Br < I.
Which alkyl halide is more reactive?
In reactions that adopt the SN1 process, 3o-alkyl halides would be more reactive than their 2o and 1o counterparts. This is contradictory to the reactivity order for the SN2 process that was observed. By either mechanism, allylic and benzylic halides are highly reactive.
Related searches to Is Br more reactive than Cl in Sn2?
- reactivity order of alkyl halides primary secondary and tertiary
- nucleophilic substitution reactions of alkyl halides examples
- sn2 reactivity order
- reactivity of alkyl halides sn1 sn2
- most reactive in sn2 reaction
- sn2 reaction of alkyl halides
- is br more reactive than cl
- alkyl halide functional group
- reactivity of alkyl halides sn1, sn2
- reactivity of alkyl halides in sn1
Information related to the topic Is Br more reactive than Cl in Sn2?
Here are the search results of the thread Is Br more reactive than Cl in Sn2? from Bing. You can read more if you want.
You have just come across an article on the topic Is Br more reactive than Cl in Sn2?. If you found this article useful, please share it. Thank you very much.