Are you looking for an answer to the topic “Is Br or Cl A stronger Nucleophile?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Why is bromide a better nucleophile than chloride? This refers to the question number #468 in 1001 in Orgo Chem Examkrackers. Generally speaking, bromide is more polarizable than chloride, improving its ability to distribute electron density unevenly around the nucleus.I⁻ is a better nucleophile than F⁻ in polar protic solvents. F⁻ is a better nucleophile than Br⁻ in polar aprotic solvents. A protic solvent has an H atom bound to O or N. It can use its H atom to participate in H-bonding with a nucleophile.Nucleophilicity increases as the density of negative charge increases. An anion is always a better nucleophile than a neutral molecule, so the conjugate base is always a better nucleophile. A highly electronegative atom is a poor nucleophile because it is unwilling to share its electrons.

Table of Contents
Is Br or nucleophilic more?
I⁻ is a better nucleophile than F⁻ in polar protic solvents. F⁻ is a better nucleophile than Br⁻ in polar aprotic solvents. A protic solvent has an H atom bound to O or N. It can use its H atom to participate in H-bonding with a nucleophile.
How do you know which nucleophile is stronger?
Nucleophilicity increases as the density of negative charge increases. An anion is always a better nucleophile than a neutral molecule, so the conjugate base is always a better nucleophile. A highly electronegative atom is a poor nucleophile because it is unwilling to share its electrons.
What Makes A Good Nucleophile? (1)
Images related to the topicWhat Makes A Good Nucleophile? (1)

Is Cl A strong or weak nucleophile?
Chad says halogens are strong nucleophiles, thus, reactions with halogens will proceed SN2. However, DAT Bootcamp says the electron on halogens are delocalized (therefore, is weakened) so Cl-, Br-, and I- are weak nucleophiles, which will make reactions go SN1.
Which is a stronger nucleophile Br or OH?
OH is a much better nucleophile than Br ; this reaction would revert if it ever happened.
Which is better leaving group Cl or Br?
Bulky groups are better leaving groups as when they leave, the steric factor gets stabilized. Br- is pretty bulky making it a better leaving group.
Is Cl A nucleophile?
As you can find in halide reactions within organic chemistry, chlorine is also a nucleophile.
Is Br an electrophile or nucleophile?
Bromine as an electrophile
Since two identical bromine atoms are joined together in the bromine molecule there is no reason why one atom should pull the bonding pair of electrons towards itself – they must be equally electronegative and so there won’t be any separation of charge, + or -.
See some more details on the topic Is Br or Cl A stronger Nucleophile? here:
Is CL a better leaving group than Br? – Cement Answers
This may be explained in part by the previous conclusion that bromide is a stronger nucleophile; it is larger than chloride, their radii being 182 pm and 167 pm …
Ch 8: Nucleophiles – Chemistry
Nucleophile means “nucleus loving” which describes the tendency of an … form the new C-X bond and increases the nucleophilicity, so I- > Br- > Cl- > F-.
Why is Cl- a better nucleophile than F-? : r/Mcat – Reddit
Cl- is a better nucleophile because its has less of a chance in picking up protons. Hmm, isn’t it opposite though? Wouldn’t it be “happier” in a …
Which of the following is correct order of nucleophilicity F Cl Br?
(F- > Cl – > Br – > I – ).
How do you find nucleophilicity order?
Within a group, the order of nucleophilicity is opposite to the order of basicity. This order of nucleophilicity is related to the polarizability of the nucleophile. The order I− > Br− > Cl− is one that we encounter many times in the study of reaction mechanisms. Another important relationship is RS− > RO−.
Is Br a weak base?
…
12.5: Strong and Weak Acids and Bases and their Salts.
| Acids | Bases |
|---|---|
| HNO3 | RbOH |
| H2SO4 | CsOH |
| HClO3 | Mg(OH)2 |
| HClO4 | Ca(OH)2 |
Is Br a good leaving group?
Good leaving groups are weak bases. They’re happy and stable on their own. Some examples of weak bases: halide ions (I-, Br-, Cl-) water (OH2), and sulfonates such as p-toluenesulfonate (OTs) and methanesulfonate (OMs).
Nucleophilicity Comparison Trick organic chemistry | IIT JEE NEET | Vineet Khatri | ATP STAR kota
Images related to the topicNucleophilicity Comparison Trick organic chemistry | IIT JEE NEET | Vineet Khatri | ATP STAR kota

What is an example of a strong nucleophile?
A good base is usually a good nucleophile. So, strong bases — substances with negatively charged O, N, and C atoms — are strong nucleophiles. Examples are: RO⁻, OH⁻, RLi, RC≡C:⁻, and NH₂⁻.
Is Cl or OH a better nucleophile?
In polar protic solvents (e.g. water and alcohols, any solvent with OH) nucleophilicity increases as you go down the periodic table (F- < Cl- < Br- < I – ). In polar aprotic solvents (e.g. DMSO, acetone) the order is reversed, and the most basic nucleophiles are also the most nucleophilic. (F- > Cl – > Br – > I – ).
Which is stronger base OH or Br?
because Br- is more stable than OH- , OH- is the conjugate base of H2O since H2O is stable . hence OH- will be weak. In case of Br- it is the conjugate base of HBr since HBr is weak hence Br- will be strong.
Which of the following has the highest nucleophilicity?
Solution : Lesser the electronegativity of the donor atom, more is its tendency to donate a pair of electron and stronger is the nucleophile. Electronegativity of C, N, 0, F are in the order `Fgt0gtNgtC`. Therefore `CH_(3)` is the strongest nucleophilic, i.e., it has highest nucleophilicity.
Is Br a weaker base than Cl?
Bases are electron donors. The strength of the base is ranked on the basis of its ability to donate electron pairs. Chlorine (Cl) is a stronger base than bromide(Br).
What is the best leaving group for nucleophilic substitution?
Alkyl chlorides are indeed common reactants in laboratory nucleophilic substitution reactions, as are alkyl bromides and alkyl iodides. Iodide, which is the least basic of the four common halides (F, Cl, Br, and I), is the best leaving group among them.
What makes something a good nucleophile?
If you read the last post, you’ll recall that a nucleophile is a species that donates a pair of electrons to form a new covalent bond. Nucleophilicity is measured by comparing reaction rates; the faster the reaction, the better (or, “stronger”) the nucleophile.
Why Cl minus is nucleophile?
Atomic chlorine have 3 lone pairs of electrons which makes it extremely electron rich. As a result it gets attracted towards positive charge making it a nucleophile.
Which one of the following is not a nucleophile Cl Br I no2+?
BF3 is electron deficient compound. It does not have lone pair of electrons to donate. So it is not nucleophilic.
Why Cl is an electrophile?
As chlorine has 7 electrons it has vacancy for 1 electron, therefore it can act as electrophile to complete its octet.
EASY TRICK TO FIND OUT WHICH IS STRONG NUCLEOPHILE AND WHICH IS WEAK NUCLEOPHILE ?
Images related to the topicEASY TRICK TO FIND OUT WHICH IS STRONG NUCLEOPHILE AND WHICH IS WEAK NUCLEOPHILE ?
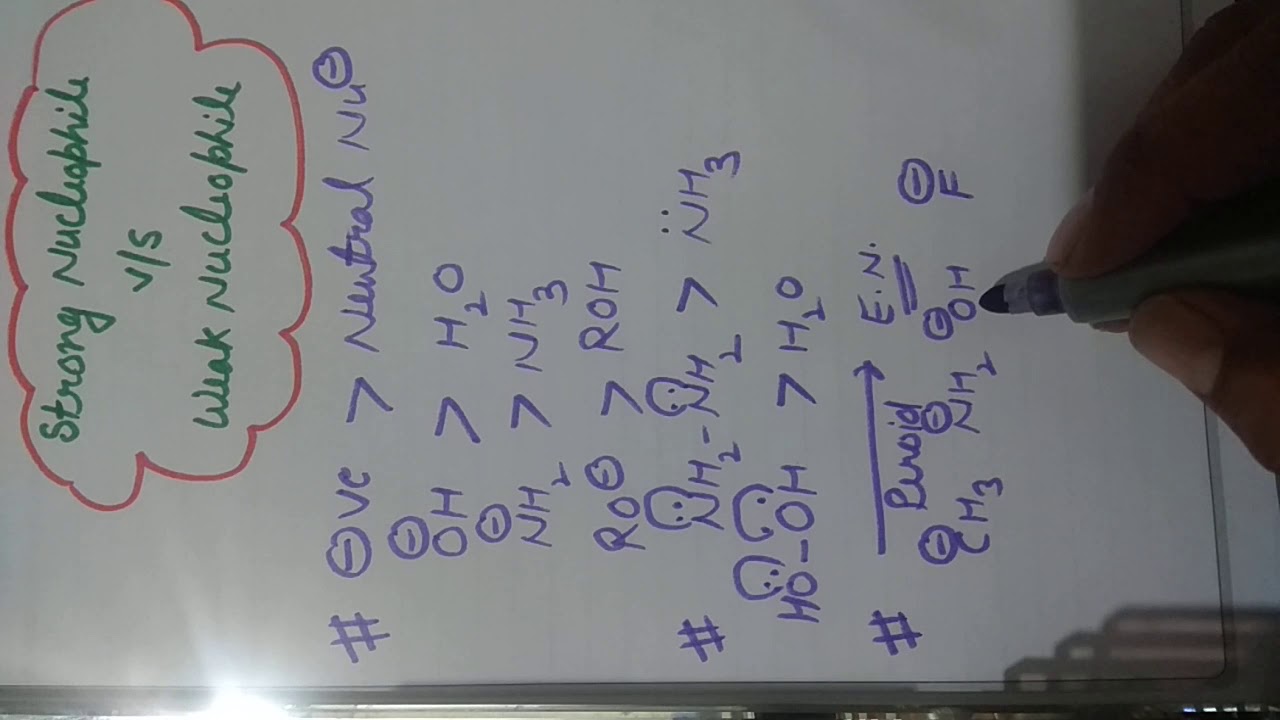
Is Cl2 a nucleophile?
Now the molecular halogens like Cl2,Br2,I2 etc accept electrons as these have vacent antibonding orbital so these are electrophile in nature . but , Cl- , Br-,I- etc have a negative charge which acted as nucleophile… So ,My ans is NO.
Which reagent is a good nucleophile?
Nh₃ (option a) is a good nucleophile compared to other mentioned in the given question. A nucleophile is a neutral or negatively charged species that tends to donate electrons to another atom to form a chemical bond. It is also called lewis base.
Related searches to Is Br or Cl A stronger Nucleophile?
- is br- a good nucleophile
- is br or cl a stronger nucleophile
- is h2o a good nucleophile
- is i or cl a better nucleophile
- is cl- a strong nucleophile
- good nucleophiles
- nucleophile strength list
- is br or cl a better leaving group
- is br or cl a better nucleophile
- is br- a strong or weak nucleophile
- good nucleophiles for sn2
- is br a good nucleophile
- why is br a good nucleophile
- is cl a strong nucleophile
- strongest nucleophile
Information related to the topic Is Br or Cl A stronger Nucleophile?
Here are the search results of the thread Is Br or Cl A stronger Nucleophile? from Bing. You can read more if you want.
You have just come across an article on the topic Is Br or Cl A stronger Nucleophile?. If you found this article useful, please share it. Thank you very much.