Are you looking for an answer to the topic “Is BrCl5 polar or nonpolar?“? We answer all your questions at the website Chiangmaiplaces.net in category: +100 Marketing Blog Post Topics & Ideas. You will find the answer right below.
Keep Reading
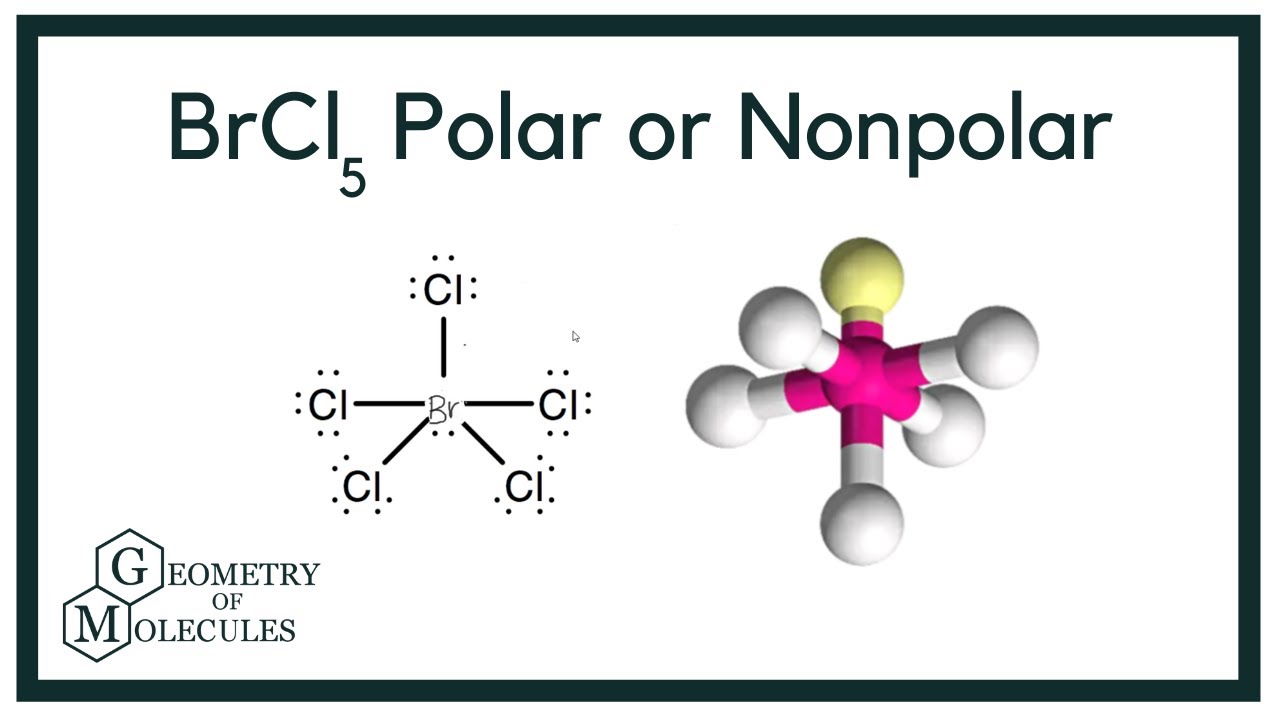
Table of Contents
Is BH3 polar?
Each B-H bond in BH3 is polar / forms a dipole because the B and H atoms have different electronegativities.
How do I know if something is polar or nonpolar?
- Draw the Lewis structure.
- Figure out the geometry (using VSEPR theory)
- Visualize or draw the geometry.
- Find the net dipole moment (you don’t have to actually do calculations if you can visualize it)
- If the net dipole moment is zero, it is non-polar. Otherwise, it is polar.
Is BrCl5 Polar or Nonpolar? (Bromine Pentachloride)
Images related to the topicIs BrCl5 Polar or Nonpolar? (Bromine Pentachloride)

Is bri5 polar or nonpolar?
Answer and Explanation: The answer is a. The molecule is polar and has polar bonds.
Is BH3 covalent or ionic?
In the molecule borane (BH₃), boron forms single covalent bonds with 3 hydrogen atoms.
Is BF3 polar molecule?
Boron trifluoride (BF3) is a nonpolar molecule, whereas ammonia (NH3) is a polar molecule.
Which of the following is non-polar?
Q. Methane molecule is non-polar molecule. Explain.
What are examples of nonpolar molecules?
- Any of the noble gasses: He, Ne, Ar, Kr, Xe (These are atoms, not technically molecules.)
- Any of the homonuclear diatomic elements: H2, N2, O2, Cl2 (These are truly nonpolar molecules.)
- Carbon dioxide – CO. …
- Benzene – C6H. …
- Carbon tetrachloride – CCl. …
- Methane – CH. …
- Ethylene – C2H.
See some more details on the topic Is BrCl5 polar or nonpolar? here:
Is BrCl5 polar or nonpolar ? – Bengis Life
Answer = BrCl5 is Nonpolar … What is polar and non-polar? Polar “In chemistry, polarity is a separation of electric charge leading to a molecule or its …
Is BrCl5 polar or nonpolar ?
BrCl5 is Nonpolar. I’ll tell you the polar or nonpolar list below. If you want to quickly find the word you want to … List molecules polar and non polar …
Is BrCl5 A Polar Molecule? – Whoat Where Why
Is BrF3 polar or nonpolar molecule? Because of the existence of two lone pairs on the central bromine atom, BrF3 (bromine …
Is BrCl5 a polar molecule? – NCERT POINT
Answer BrCl5 (bromine pentachloride) is sometimes referred to as a polar molecule and other times as a nonpolar molecule.
Which molecule is nonpolar?
Nonpolar Molecule Examples
Examples of homonuclear nonpolar molecules are oxygen (O2), nitrogen (N2), and ozone (O3). Other nonpolar molecules include carbon dioxide (CO2) and the organic molecules methane (CH4), toluene, and gasoline. Most carbon compounds are nonpolar.
Is ClO3 ionic?
Chlorates are inorganic salts of chloric acid that contain the ClO3- ion.
What is the bond angle of ClO3?
Based on Lewis Dot structure of ClO 3 – , the shape of ClO 3 – is trigonal pyramidal and there are 3 bond pairs and 1 lone pair. This one lone pair of electrons exerts a big repulsive force on adjacent bond pairs to compress bond angles to make them smaller than 109.5 o and it is 107 o.
What is the shape of ClO3?
According to VSEPR theory, ClO3– ion has pyramidal geometry due to the presence of one lone pair and three σ bond pairs which result in sp3 hybridisation.
Determine whether each molecule is polar or nonpolar a SCl2 b SCl4 c BrCl5
Images related to the topicDetermine whether each molecule is polar or nonpolar a SCl2 b SCl4 c BrCl5

Is SCl4 polar?
SCl4 is a polar molecule because of asymmetrical geometry that causes the non-uniform distribution of charge in the molecule. In the SCl4 lewis structure, a total of 13 lone pairs and 4 bond pairs are present.
Is SeF4 polar?
So, Is SeF4 polar or nonpolar? SeF4 is a polar molecule because of its asymmetrical structure which causes uneven distribution of charges in the molecule, thus, dipole moments generated along with the bonds are unable to cancel out each other, making SeF4 a polar molecule in nature.
Is so32 polar or nonpolar?
Answer: SO3(2-) is a polar molecule due to the lone pair electrons at the “top” of the structure causing electron-electron repulsion and a region of partial negative charge.
Does BH3 dipole bond?
The bonds in BH3 will therefore be somewhat polarized, with the local dipoles oriented towards the hydrogen atoms, as shown below. But because the molecule is symmetrical, the three dipole arrows cancel and, as a molecule, BH3 has no net molecular dipole.
What kind of compound is BH3?
BH3 or known as borane is an covalent compound as it is formed by sharing of electrons.
What intermolecular force is BH3?
borane (BH3) dipole-dipole forces. 2. They have similar molecular weights: Br₂, 160; ICI, 162.
Why is BF3 polar or nonpolar?
BF3 (Boron Trifluoride) is Non-Polar because of its highly symmetric shape. It has a Trigonal Planar geometry which cancels out the dipole moments of the three BF bonds making the resultant Dipole Moment of the compound equal to 0 (Zero).
Is BF3 polar or nonpolar and justify?
BF3 is a non-polar compound. In BF3, the central boron atom has sp2 hybridized orbitals, resulting in an unfilled p orbital on the Bron atom and trigonal planar molecular geometry. Because the Boron-Fluorine bonds are all 120 degrees apart, any net dipole in that plane is canceled out.
Why BF3 is non polar explain?
Because BF3 has symmetrical shape the net dipole moment is zero and thus it is non – polar.
Which of the following is a non-polar bond?
The answer is a.
The bond between two atoms of fluorine in the diatomic fluorine gas molecule is a nonpolar covalent bond.
Polar and NonPolar Molecules: How To Tell If a Molecule is Polar or Nonpolar
Images related to the topicPolar and NonPolar Molecules: How To Tell If a Molecule is Polar or Nonpolar

Which of the following molecules is non-polar * 1 point HF H₂o Nh₃ Ch₄?
Answer: Ch4 and Nh4 are the non palar molecules..
Which of the following molecules is nonpolar Mcq?
Solution : Carbon tetrachloride, `C Cl_(4)` is a non-polar molecule.
Related searches to Is BrCl5 polar or nonpolar?
- is so2 polar or nonpolar
- brcl5 molecular geometry
- is brcl5 polar or nonpolar
- is xef4 polar or nonpolar
- is ses3 polar or nonpolar
- is brcl5 polar
- is brcl5 polar or nonpolar molecule
- is pobr3 polar or nonpolar
- is shf polar or nonpolar
- brcl5 intermolecular forces
- is co2 polar or nonpolar
- is np polar or nonpolar
- brcl5 lewis structure
- is nocl polar or nonpolar
- is o-n polar or nonpolar
- is cs2 polar or nonpolar
- is ko polar or nonpolar
- scl4 polar or nonpolar
Information related to the topic Is BrCl5 polar or nonpolar?
Here are the search results of the thread Is BrCl5 polar or nonpolar? from Bing. You can read more if you want.
You have just come across an article on the topic Is BrCl5 polar or nonpolar?. If you found this article useful, please share it. Thank you very much.